Abstract
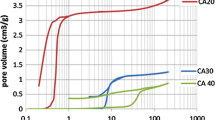
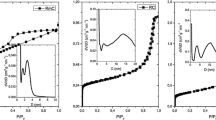
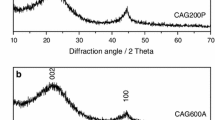
Nitrogen-containing carbon aerogel was prepared from resorcinol–melamine–formaldehyde (R–M–F) polymer gel precursor. The polymer gel was supercritically dried with CO2, and the carbonization of the resulting polymer aerogel under nitrogen atmosphere at 900 °C yielded the carbon aerogel. The polymer and carbon aerogels were characterized with TG/DTA–MS, low-temperature nitrogen adsorption/desorption (− 196 °C), FTIR, Raman, powder XRD and SEM–EDX techniques. The thermal decomposition of the polymer aerogel had two major steps. The first step was at 150 °C, where the unreacted monomers and the residual solvent were released, and the second one at 300 °C, where the species belonging to the polymer network decomposition could be detected. The pyrolytic conversion of the polymer aerogel was successful, as 0.89 at.% nitrogen was retained in the carbon matrix. The nitrogen-doped carbon aerogel was amorphous and possessed a hierarchical porous structure. It had a significant specific surface area (890 m2 g−1) and pore volume (4.7 cm3 g−1). TG/DTA–MS measurement revealed that during storage in ambient conditions surface functional groups formed, which were released upon annealing.









Similar content being viewed by others
References
Hu P, Tan B, Long M. Advanced nanoarchitectures of carbon aerogels for multifunctional environmental applications. Nanotechnol. Rev. 2016;5:23–39.
Moreno-Castilla C, Maldonado-Hódar FJ. Carbon aerogels for catalysis applications: an overview. Carbon. 2005;43:455–65.
Veselá P, Slovák V. Organic xerogels based on condensation of different m-substituted phenols with formaldehyde: Preparation and TG-MS study. J Therm Anal Calorim. 2014;116:663–9.
Jin M, Luo N, Li G, Luo Y. The thermal decomposition mechanism of nitrocellulose aerogel. J Therm Anal Calorim. 2015;121:901–8.
Horvat G, Fajfar T, Perva Uzunalić A, Knez Ž, Novak Z. Thermal properties of polysaccharide aerogels. J Therm Anal Calorim. 2017;127:363–70.
Schwan M, Ratke L. Flexible carbon aerogels. C. 2016;2:22.
Nagy B, Villar-Rodil S, Tascón JMD, Bakos I, László K. Nitrogen doped mesoporous carbon aerogels and implications for electrocatalytic oxygen reduction reactions. Microporous Mesoporous Mater. 2016;230:135–44.
Tian HY, Buckley CE, Wang SB, Zhou MF. Enhanced hydrogen storage capacity in carbon aerogels treated with KOH. Carbon. 2009;47:2128–30.
Wang X, Liu L, Wang X, Bai L, Wu H, Zhang X, et al. Preparation and performances of carbon aerogel microspheres for the application of supercapacitor. J Solid State Electrochem. 2011;15:643–8.
Zhang S, Gross AF, Van Atta SL, Lopez M, Liu P, Ahn CC, et al. The synthesis and hydrogen storage properties of a MgH2 incorporated carbon aerogel scaffold. Nanotechnology, vol. 20. Bristol: IOP Publishing; 2009. p. 204027.
Pekala RW, Farmer JC, Alviso CT, Tran TD, Mayer ST, Miller JM, et al. Carbon aerogels for electrochemical applications. J Non Cryst Solids. 1998;225:74–80.
Wang Y, Chang B, Guan D, Dong X. Mesoporous activated carbon spheres derived from resorcinol-formaldehyde resin with high performance for supercapacitors. J Solid State Electrochem. 2015;19:1783–91.
Tsujimura S, Kamitaka Y, Kano K. Diffusion-controlled oxygen reduction on multi-copper oxidase-adsorbed carbon aerogel electrodes without mediator. Fuel Cells. 2007;7:463–9.
Pekala RW. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J Mater Sci. 1989;24:3221–7.
Jin Y, Wu M, Zhao G, Li M. Photocatalysis-enhanced electrosorption process for degradation of high-concentration dye wastewater on TiO2/carbon aerogel. Chem Eng J. 2011;168:1248–55.
Geng D, Chen YY, Chen YY, Li Y, Li R, Sun X, et al. High oxygen-reduction activity and durability of nitrogen-doped graphene. Energy Environ Sci. 2011;4:760.
Long D, Zhang J, Yang J, Hu Z, Cheng G, Liu X, et al. Chemical state of nitrogen in carbon aerogels issued from phenol-melamine-formaldehyde gels. Carbon. 2008;46:1259–62.
Rasines G, Lavela P, Macías C, Zafra MC, Tirado JL, Parra JB, et al. N-doped monolithic carbon aerogel electrodes with optimized features for the electrosorption of ions. Carbon. 2015;83:262–74.
Veselá P, Slovák V. Pyrolysis of N-doped organic aerogels with relation to sorption properties. J Therm Anal Calorim. 2012;108:475–80.
Liu X, Li S, Mei J, Lau W-M, Mi R, Li Y, et al. From melamine–resorcinol–formaldehyde to nitrogen-doped carbon xerogels with micro-and meso-pores for lithium batteries. J Mater Chem A. 2014;2:14429–38.
Jin H, Zhang H, Zhong H, Zhang J, Mukundan R, Garland N, et al. Nitrogen-doped carbon xerogel: a novel carbon-based electrocatalyst for oxygen reduction reaction in proton exchange membrane (PEM) fuel cells. Energy Environ Sci R Soc Chem. 2011;4:3389.
Nagy B, Villar-Rodil S, Tascón JMD, Bakos I, László K. Nitrogen doped mesoporous carbon aerogels and implications for electrocatalytic oxygen reduction reactions. Microporous Mesoporous Mater. 2016;230:135–44.
Veselá P, Slovák V. Monitoring of N-doped organic xerogels pyrolysis by TG-MS. J Therm Anal Calorim. 2013;113:209–17.
Brunauer S, Emmett PH, Teller E. Adsorption of gases in multimolecular layers. J Am Chem Soc. 1938;60:309–19.
Dubinin MM, Radushkevich LV. The equation of the characteristic curve of activated charcoal. Dokl Akad Nauk SSSR. 1947;55:327–9.
Françoise Rouquerol GMJRKSWS. Adsorption by powders and porous solids, second edition: principles, methodology and applications [Internet]. Acad. Press. 1999 [cited 2017 May 22]. p. 647. Available from: https://books.google.hu/books?hl=en&lr=&id=UOE-ZscCYncC&oi=fnd&pg=PP1&dq=Adsorption+by+Powders+and+Porous+Solids&ots=0R1XIBpnix&sig=KX9hHmrIFZSewWz4wsxWBDusDsg&redir_esc=y#v=onepage&q=Adsorption by Powders and Porous Solids&f = false.
Al-Muhtaseb SA, Ritter JA. Preparation and properties of resorcinol-formaldehyde organic and carbon gels. Adv Mater. 2003;15:101–14.
Kim S, Kim HJ. Effect of addition of polyvinyl acetate to melamine-formaldehyde resin on the adhesion and formaldehyde emission in engineered flooring. Int J Adhes Adhes. 2005;25:456–61.
Ullah S, Bustam MA, Nadeem M, Naz MY, Tan WL, Shariff AM. Synthesis and thermal degradation studies of melamine formaldehyde resins. Sci World J. 2014;19:225.
Macias C, Rasines G, García T, Zafra M, Lavela P, Tirado J, et al. Synthesis of porous and mechanically compliant carbon aerogels using conductive and structural additives. Gels. 2016;2:4.
Zhou HH, Xu S, Su HP, Wang M, Qiao WM, Ling LC, et al. Facile preparation and ultra-microporous structure of melamine-resorcinol-formaldehyde polymeric microspheres. Chem Commun. 2013;49:3763–5.
Acknowledgements
I. M. Szilágyi thanks for a János Bolyai Research Fellowship of the Hungarian Academy of Sciences and an ÚNKP-17-4-IV-BME-188 grant supported by the ÚNKP-17-4-IV New National Excellence Program of the Ministry of Human Capacities, Hungary. The research within Project No. VEKOP-2.3.2-16-2017-00013 was supported by the European Union and the State of Hungary, co-financed by the European Regional Development Fund. An OTKA 109558 grant, an NRDI K 124212 grant and an NRDI TNN_16 123631 grant are acknowledged. The authors are grateful to Mr. György Bosznai for the technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bakos, L.P., Mensah, J., László, K. et al. Preparation and characterization of a nitrogen-doped mesoporous carbon aerogel and its polymer precursor. J Therm Anal Calorim 134, 933–939 (2018). https://doi.org/10.1007/s10973-018-7318-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7318-4