Abstract
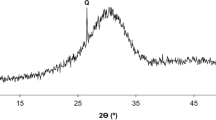
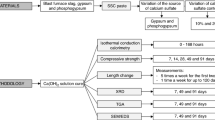
Considerable attention has been given to special cements, capable of reducing CO2 emissions, energy and limestone consumption. Supersulfated cements are made of blast furnace slag (GBFS), calcium sulfate (CS), and small quantities of activator, but achieving their optimal proportions is complex. In this paper, the effects of the both CS and alkali activator (KOH) contents were studied. The main results showed that the compressive strength, heat of hydration, and consumption of anhydrite phase were strongly influenced by the alkaline content, while low calcium sulfate or alkaline content increased the formation of CSH. The instability of ettringite was verified: with low CS, the probable hypothesis was its conversion into monosulfate due to the scarcity of sulfate; with high CS, it was associated with intense, rapid consumption of anhydrite with high KOH content, followed by the precipitation of ettringite on the surface of slag grains and its conversion into monosulfate.









Similar content being viewed by others
References
Gruskovnjak A, Lothenbach B, Winnefeld F, Figi R, Ko S-C, Adler M, Mäder U. Hydration mechanisms of super sulphated slag cement. Cem Concr Res. 2008;38:983–92.
Matschei T, Bellmann F, Stark J. Hydration behaviour of sulphate-activated slag cements. Adv Cem Res. 2005;17:167–78.
Juenger MCG, Winnefeld F, Provis JL, Ideker JH. Advances in alternative cementitious binders. Cem Concr Res. 2011;41:1232–43.
Grounds Z, Nowell DV, Wilburn FW. The influence of temperature and different storage conditions on the stability of supersulphated cement. J Therm Anal. 1994;41:687–99.
Grounds Z, Nowell DV, Wilburn FW. Resistance of supersulphated cement to strong sulfate solutions. J Therm Anal Calorim. 2003;72:181–90.
Angulski da Luz C, Hooton RD. Influence of curing temperature on the process of hydration of supersulfated cements at early age. Cem Concr Res. 2015;77:69–75.
Collier NC, Milestone NB, Gordon LE, Ko SC. The suitability of a supersulfated cement for nuclear waste immobilization. J Nucl Mater. 2014;452:457–64.
Gracioli B, Varella M, Buth IS, Angulski da Luz C, Pereira Filho JI. Valorization of galvanic sludge and phosphogypsum in supersulfated cement (CCS). In: Proceedings of the 3rd International conference on sustainable solid waste management, Tinos island, Greece; 2015.
EN 15743, Supersulfated cement—composition, specification and conformity criteria, European Committee for Standardization (CEN), Brussels, Belgium, 2009.
Dutta DK, Borthakur PC. Activation of low lime high alumina granulated blast furnace slag by anhydrite. Cem Concr Res. 1990;20:711–22.
Mun KJ, Hyoung WK, Lee CW, So SY, Soh YS. Basic properties of non-sintering cement using phosphogypsum and waste lime as activator. Constr Build Mater. 2007;21:1342–50.
Erdem E, Olmez H. The mechanical properties of supersulfated cement containing phosphogypsum. Cem Concr Res. 1993;23:115–21.
Bijen J, Niel E. Supersulphated cement from blast furnace slag and gypsum available in the Netherlands and neighbouring countries. Cem Concr Res. 1981;11:307–22.
Acknowledgements
The authors wish to thank the CNPq (National Counsel of Technological and Scientific Development, grant number 483661/2013-9) and the CAPES (Coordination for the Improvement of Higher Education Personnel) in Brazil for their support, together with the Natural Sciences and Research Council of Canada (grant number RGPIN 108067).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rubert, S., Angulski da Luz, C., F. Varela, M.V. et al. Hydration mechanisms of supersulfated cement. J Therm Anal Calorim 134, 971–980 (2018). https://doi.org/10.1007/s10973-018-7243-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7243-6