Abstract
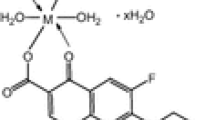
New mixed-ligand Co(II) and Ni(II) complexes with enrofloxacin and 1,10-phenanthroline/8-hydroxyquinoline have been synthesized. The metal complexes have been characterized by elemental analyses, molar conductance, FT-IR, solid reflectance, magnetic moment, thermal analyses, X-ray powder diffraction (XRD) and SEM analysis. Conductance measurements indicate that all the complexes are non-electrolytes. Spectral IR data showed that the deprotonated enrofloxacin is bidentately bound to the metal ion through the pyridone oxygen and the carboxylated oxygen; 1,10-phenanthroline acts as neutral bidentate ligand coordinated through two nitrogen donor atoms, and the deprotonated 8-hydroxyquinoline is coordinated through nitrogen and oxygen donor atoms. The geometry of the complexes was elucidated with solid reflectance UV and magnetic moments. Thermal analysis recorded that TG/DTG/DSC experiments revealed the thermal stability of metal complexes and confirmed their compositions suggested by the analytical data. In addition to experimental data, a molecular modelling study has been performed to establish the optimized geometries of metal complexes. The XRD analysis of powders evidences the nanocrystalline nature of all complexes. The results are in agreement with molecular modelling data. SEM images revealed highly agglomerated particles with irregular shape with sizes in the micrometre range. The ligand and their metal complexes were screened for their antimicrobial activity against Escherichia coli 25922, Staphylococcus aureus 25923, Pseudomonas aeruginosa 27853 and Candida albicans 10231. The metal complexes were also investigated for their cytotoxicity using an in vitro assay.









Similar content being viewed by others
References
Suk JE, Semenza JC. Future infectious disease threats to Europe. Am J Pub Health. 2011;101:2068–79.
Popa M, Hussien MD, Cirstea A, Lazar V, Bezirtzoglou E, Chifiriuc MC, Sakizlian M, Stavropoulou E, Bertesteanu S. Insights on metal based dental implants and their interaction with the surrounding tissues. Curr Top Med Chem. 2015;15:1614–21.
Bertesteanu SVG, Popescu CR, Grigore R, Popescu B. Pharyngoesophageal junction neoplasia–therapeutic management. Chirurgia. 2012;107:33–8.
Aljahdali M, EL-Sherif AA. Synthesis, characterization, molecular modelling and biological activity of mixed ligand complexes of Cu(II), Ni(II) and Co(II) based on 1,10-phenantroline and novel thiosemicarbazone. Inorg Chim Acta. 2013;407:58–68.
Agwara MO, Ndifon PT, Ndosiri NB, Paboudam AG, Yufanyi DM, Mohamadou A. Synthesis, characterisation and antimicrobial activities of cobalt(II), copper(II) and zinc(II) mixed-ligand complexes containing 1,10-phenanthroline and 2,2′-bipyridine. Bull Chem Soc Ethiopia. 2010;24:383–9.
Patil SS, Thakur GA, Shaikh MM. Synthesis, spectral, thermal and antibacterial investigations of mixed ligand complexes of thorium(IV) derived from 8-hydroxyquinoline and some amino acids. Acta Pol Pharm Drug Res. 2012;69:1087–93.
Saha DK, Sandbhor U, Shirisha K, Padhye S, Deobagkar D, Ansond CE, Powell AK. A novel mixed-ligand antimycobacterial dimeric copper complex of ciprofloxacin and phenanthroline. Bioorg Med Chem Lett. 2004;14:3027–32.
Irgi E, Geromichalos G, Balala S, Kljun J, Kalogiannis S, Papadopoulos A, Turel I, Psomas G. Cobalt(II) complexes with the quinolone antimicrobial drug oxolinic acid: structure and biological perspectives. RSC Adv. 2015;5:36353–67.
Dhanaraj CJ, Johnson J. Quinoxaline based bio-active mixed ligand transition metal complexes: synthesis, characterization, electrochemical, antimicrobial, DNA binding, cleavage, antioxidant and molecular docking studies. J Photochem Photobiol B. 2015;151:100–9.
Darawsheh M, Abu Ali H, Abuhijleh AL, Rappocciolo E, Akkawi M, Jaber S, Maloul S, Hussein Y. New mixed ligand zinc(II) complexes based on the antiepileptic drug sodium valproate and bioactive nitrogen-donor ligands. Synthesis, structure and biological properties. Eur J Med Chem. 2014;82:152–63.
Protogeraki C, Andreadou EG, Perdih F, Turel I, Pantazaki AA, Psomas G. Cobalt(II) complexes with the antimicrobial drug enrofloxacin: structure, antimicrobial activity, DNA and albumin-binding. Eur J Med Chem. 2014;86:189–201.
Farrell N. Transition metal complexes as drugs and chemotherapeutic agents. Dordrecht: Kluwer; 1989.
Turel I. The interactions of metal ions with quinolone antibacterial agents. Coord Chem Rev. 2002;232:27–47.
Uivarosi V. Metal complexes of quinolone antibiotics and their applications: an update. Molecules. 2013;18:11153–97.
Reiss R, Florea S, Căproiu T, Stănica N. Synthesis, characterization and antibacterial studies of some transition metals with the Schiff base N-(2-Furanylmethylene)-3-aminodibenzofuran. Turkish J Chem. 2009;33:775–83.
Reiss A, Mureseanu M. Transition metal complexes with ligand containing thioamide moiety: synthesis, characterization and antibacterial activity. J Chil Chem Soc. 2012;57:1199–204.
Reiss A, Chifiriuc MC, Amzoiu E, Spînu CI. Transition METAL(II) complexes with cefotaxime-derived schiff base: synthesis, characterization and antimicrobial studies. Bioinorg Chem Appl. 2014, Article ID 926287.
Reiss A, Samide A, Ciobanu G, Dăbuleanu I. Synthesis, spectral characterization and thermal behaviour of new metal (II) complexes with Schiff Base derived from amoxicillin. J Chil Chem Soc. 2015;60:3074–9.
Constantinescu CD, Rotaru A, Nedelcea A, Dinescu M. Thermal behaviour and matrix-assisted pulsed laser evaporation deposition of functional polymeric materials thin films with potential use in optoelectronics. Mat Sci Semicond Proc. 2015;30:242–9.
Rotaru A. Thermal and kinetic study of hexagonal boric acid versus triclinic boric acid in air flow. J Therm Anal Calorim. 2017;127:755–63.
Rotaru A, Dumitru M. Thermal behaviour of CODA azoic dye liquid crystal and nanostructuring by drop cast and spin coating techniques. J Therm Anal Calorim. 2017;127:21–32.
Rotaru A. Discriminating within the kinetic models for heterogeneous processes of materials by employing a combined procedure under TKS-SP 2.0 software. J Therm Anal Calorim. 2016;126:919–32.
Krajnikova A, Rotaru A, Gyoryova K, Homzova K, Manolea HO, Kovarova J, Hudecova D. Thermal behaviour and antimicrobial assay of some new zinc(II) 2-aminobenzoate complex compounds with bioactive ligands. J Therm Anal Calorim. 2015;120:73–83.
Rotaru A, Gosa M. Computational thermal and kinetic analysis. Complete standard procedure to evaluate the kinetic triplet form non-isothermal data. J Therm Anal Calorim. 2015;120:73–83.
Rotaru A, Bratulescu G, Rotaru P. Thermal analysis of azoic dyes: part I. Non-isothermal decomposition kinetics of [4-(4-chlorobenzyloxy)-3-methylphenyl](p-tolyl)diazene in dynamic air atmosphere. Thermochim Acta. 2009;489:63–9.
Rotaru A, Gosa M, Rotaru P. Computational thermal and kinetic analysis. Software for non-isothermal kinetics by standard procedure. J Therm Anal Calorim. 2008;94:367–71.
Rotaru A. Thermal analysis and kinetic study of Petrosani bituminous coal from Romania in comparison with a sample of Ural bituminous coal. J Therm Anal Calorim. 2012;110:1283–91.
Roisinel T, Rodriguez-Carvajal J. A windows tool for powder diffraction patterns analysis. In: Proceedings of the seventh European powder diffraction conference (EPDIC 7), 2000, p. 118. See also http://www-llb.cea.fr/Fullweb/winplotr/winplotr.htm.
Olar R, Badea M, Marinescu D, Chifiriuc MC, Bleotu C, Grecu MN, Iorgulescu E, Bucur M, Lazar V, Finaru A. Prospects for new antimicrobials based on N,N-dimethylbiguanide complexes as effective agents on both planktonic and adhered microbial strains. Eur J Med Chem. 2010;45:2868–75.
Limban C, Chifiriuc MC. Antibacterial activity of new dibenzoxepinone oximes with fluorine and trifluoromethyl group substituents. Int J Mol Sci. 2011;12:6432–44.
Nica IC, Stan MS, Dinischiotu A, Popa M, Chifiriuc MC, Lazar V, Pircalabioru GG, Bezirtzoglou E, Iordache OG, Varzaru E, Dumitrescu I, Feder M, Vasiliu F, Mercioniu I, Diamandescu L. Innovative self-cleaning and biocompatible polyester textiles nano-decorated with Fe–N-doped titanium dioxide. Nanomaterials. 2016;6:214–20.
Geary WJ. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev. 1971;7:81–122.
Deacon GB, Phillips RJ. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord Chem Rev. 1980;33:227–50.
Chalkidou E, Perdih F, Turel I, Kessissollou DP, Psomas G. Copper(II) complexes with antimicrobial drug flumequine: structure and biological evaluation. J Inorg Biochem. 2012;113:55–65.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds. 4th ed. New York: Wiley; 1986.
Efthimiadou EK, Katsaros N, Karaliota A, Psomas G. Mononuclear copper(II) complexes with quinolones and nitrogen-donor heterocyclic ligands: synthesis, characterization, biological activity and interaction with DNA. Inorg Chim Acta. 2007;360:4093–102.
Hosny WM. Dioxouranium (VI) mixed ligand complexes containing 8-hydroxyquinoline and some amino acids. Synth React Inorg Met Org Chem. 1998;28:1029–52.
Abou Sekkina MM, El Helbawy SM. Vibrational spectra of some solid 8-hydroxyquinoline metal complexes in correlation with their coordination bond length and type of metal ion. Proc Indian Sci Acad. 1985;51A:959–64.
Lever ABP. Crystal field spectra. Inorganic electronic spectroscopy. 1st ed. Amsterdam: Elsevier; 1968.
Cotton FA, Wilkinson G, Murillo CA, Bochman M. Advanced inorganic chemistry. 6th ed. New York: Wiley; 2003.
Reiss A, Chifiriuc MC, Amzoiu E, Cioateră N, Dabuleanu I, Rotaru P. New metal(II) complexes with ceftazidime Schiff base. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s10973-017-6832-0.
Rotaru P, Scorei R, Harabor A, Dumitru MD. Thermal analysis of a calcium fructoborate sample. Thermochim Acta. 2010;506:8–13.
Tatucu M, Rotaru P, Rau I, Spinu C, Kriza A. Thermal behaviour and spectroscopic investigation of some methyl 2-pyridyl ketone complexes. J Therm Anal Calorim. 2010;100:1107–14.
Constantinescu C, Morintale E, Ion V, Moldovan A, Luculescu C, Dinescu M, Rotaru P. Thermal, morphological and optical investigations of Cu(DAB)2 thin films produced by matrix-assisted pulsed laser evaporation and laser-induced forward transfer for sensor development. Thin Solid Films. 2012;520:3904–9.
Popescu M, Rotaru P, Bubulica MV, Kriza A. New complexes with 2-pyridyl ketone Schiff bases. Synthesis, structural analysis and thermal studies. J Therm Anal Calorim. 2015;120:641–52.
Rotaru A, Constantinescu C, Mandruleanu A, Rotaru P, Moldovan A, Gyoryova K, Dinescu M, Balek V. Matrix assisted pulsed laser evaporation of zinc benzoate for ZnO thin films and non-isothermal decomposition kinetics. Thermochim Acta. 2010;498:81–91.
Constantinescu C, Morintale E, Emandi A, Dinescu M, Rotaru P. Thermal and microstructural analysis of Cu(II) 2,2′-dihydroxy azobenzene and thin films deposition by MAPLE technique. J Therm Anal Calorim. 2011;104:707–16.
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL. General atomic and molecular electronic structure system. J Comput Chem. 1993;14:1347–63.
Chihaia V, Sutmann G, Lee CS, Suh SH. Divergence-free description for molecular rotation in cartesian coordinates: the axis-rotation formula and some of its applications to computational chemistry. Rev Roum Chim. 2007;52:795–808.
Skyrianou KC, Psycharis V, Raptopoulou CP, Kessissoglou DP, Psomas G. Nickel-quinolone interaction. Part 4—structure and biological evaluation of nickel (II)—enrofloxacin complexes compared to zinc (II) analogues. J Inorg Biochem. 2011;105:63–74.
Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976;A32:751–67.
Trouchon T, Lefebvre S. A review of enrofloxacin for veterinary use. Open J Vet Med. 2016;6:40–58.
Levine C, Hiasa H, Marians KJ. DNA gyrase and topoisomerase IV: biochemical activities, physiological roles during chromosome replication, and drug sensitivities. Biochim Biophys Acta. 1998;1400:29–43.
Tweedy BG. Plant extracts with metal ions as potential antimicrobial agents. Phytopathology. 1964;55:910–4.
Soliman MH, Mohamed GG, Hindy AMM. Biological activity, spectral and thermal characterization of mixed ligand complexes of enrofloxacin and glycine: in vitro antibacterial and antifungal activity studies. Monatsh Chem. 2015;146:259–73.
Silva PP, Guerra W, Silveira JN, Ferreira AM, Bortolotto T, et al. Two new ternary complexes of copper(II) with tetracycline or doxycyline and 1,10-phenanthroline and their potential as antitumoral: cytotoxicity and DNA cleavage. Inorg Chem. 2011;50:6414–24.
Pivetta T, Isaia F, Verani G, Cannas C, Serra L, Castellano C, Demartin F, Pilla F, Manca M, Pani A. Mixed-1,10-phenanthroline-Cu(II) complexes: synthesis, cytotoxic activity versus hematological and solid tumour cells and complex formation equilibria with glutathione. J Inorg Biochem. 2012;114:28–37.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reiss, A., Cioatera, N., Chifiriuc, M.C. et al. New biologically active mixed-ligand Co(II) and Ni(II) complexes of enrofloxacin. J Therm Anal Calorim 134, 527–541 (2018). https://doi.org/10.1007/s10973-018-6994-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-6994-4