Abstract
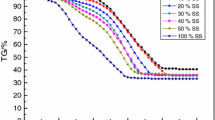
The co-combustion characteristics of municipal sewage sludge and bituminous coal in air and O2/CO2 atmospheres were assessed by using a thermogravimetric analysis approach between heating rates of 20 and 80 K min−1. The combustion characteristics of sewage sludge/bituminous coal blend (50% blending ratio) in air were also compared with its oxy-fuel behavior. In air atmosphere, the ignition temperatures for sludge/coal blends were between those for their parent samples, which ranged from 238 to 418 °C. In O2/CO2 atmosphere with oxygen content of 30%, the ignition temperatures of 50%sewage sludge/50%coal blend were between 260 and 275 °C, which were less than those (268–280 °C) in air. The ignition and burnout indexes of the sample in 30%O2/70%CO2 atmosphere were very close to those in air. The temperatures with respect to maximum combustion rates of the 50%sewage sludge/50%coal blend in 40%O2/60%CO2 were lower than those in air. The average value of the activation energy for the 50%sewage sludge/50%coal blend in 30%O2/70%CO2 was less than that in air.







Similar content being viewed by others
Abbreviations
- α :
-
Conversion degree
- A :
-
Pre-exponential factor (s−1)
- C:
-
Char
- D :
-
Combustion index
- E a :
-
Apparent activation energy (J mol−1)
- m :
-
Mean; empirical constant in integral function
- n :
-
Reaction order
- R :
-
Universal gas constant (J mol−1K−1)
- T :
-
Temperature (K/ °C)
- VM:
-
Volatile matter
- β :
-
Heating rate (K min−1)
- b:
-
Burnout
- i:
-
Ignition
- max:
-
Maximum
- p:
-
Peak
- ∆:
-
Increment
References
Fytili D, Zabaniotou A. Utilization of sewage sludge in EU application of old and new methods—a review. Renew Sustain Energy Rev. 2008;12(1):116–40. doi:10.1016/j.rser.2006.05.014.
He Q, Xie D, Xu R, Wang T, Hu B. The utilization of sewage sludge by blending with coal water slurry. Fuel. 2015;159:40–4. doi:10.1016/j.fuel.2015.06.071.
Chen H, Yan S-H, Ye Z-L, Meng H-J, Zhu Y-G. Utilization of urban sewage sludge: Chinese perspectives. Environ Sci Pollut R. 2012;19(5):1454–63. doi:10.1007/s11356-012-0760-0.
Duan F, Zhang L, Sun X, Huang Y. Comparison of thermal behavior for modified calcium magnesium acetate blended separately with peanut shell and sewage sludge at different atmospheres. J Therm Anal Calorim. 2017;127(3):2417–25. doi:10.1007/s10973-016-5829-4.
Tyagi VK, Lo S-L. Sludge: a waste or renewable source for energy and resources recovery? Renew Sustain Energy Rev. 2013;25:708–28. doi:10.1016/j.rser.2013.05.029.
Xiao H, Ma X, Liu K. Co-combustion kinetics of sewage sludge with coal and coal gangue under different atmospheres. Energy Convers Manage. 2010;51(10):1976–80. doi:10.1016/j.enconman.2010.02.030.
Coimbra RN, Paniagua S, Escapa C, Calvo LF, Otero M. Combustion of primary and secondary pulp mill sludge and their respective blends with coal: a thermogravimetric assessment. Renew Energy. 2015;83:1050–8. doi:10.1016/j.renene.2015.05.046.
McIlveen-Wright DR, Huang Y, Rezvani S, Wang Y. A technical and environmental analysis of co-combustion of coal and biomass in fluidised bed technologies. Fuel. 2007;86(14):2032–42. doi:10.1016/j.fuel.2007.02.011.
Tsai M-Y, Wu K-T, Huang C-C, Lee H-T. Co-firing of paper mill sludge and coal in an industrial circulating fluidized bed boiler. Waste Manage. 2002;22(4):439–42. doi:10.1016/S0956-053X(02)00027-2.
Vamvuka D, Salpigidou N, Kastanaki E, Sfakiotakis S. Possibility of using paper sludge in co-firing applications. Fuel. 2009;88(4):637–43. doi:10.1016/j.fuel.2008.09.029.
Areeprasert C, Scala F, Coppola A, Urciuolo M, Chirone R, Chanyavanich P, et al. Fluidized bed co-combustion of hydrothermally treated paper sludge with two coals of different rank. Fuel Process Technol. 2016;144:230–8. doi:10.1016/j.fuproc.2015.12.033.
Buhre BJP, Elliott LK, Sheng CD, Gupta RP, Wall TF. Oxy-fuel combustion technology for coal-fired power generation. Prog Energy Combust. 2005;31(4):283–307. doi:10.1016/j.pecs.2005.07.001.
Escudero AI, Espatolero S, Romeo LM, Lara Y, Paufique C, Lesort A-L, et al. Minimization of CO2 capture energy penalty in second generation oxy-fuel power plants. Appl Therm Eng. 2016;103:274–81. doi:10.1016/j.applthermaleng.2016.04.116.
Yuzbasi NS, Selçuk N. Air and oxy-fuel combustion behaviour of petcoke/lignite blends. Fuel. 2012;92(1):137–44. doi:10.1016/j.fuel.2011.08.026.
Gładysz P, Ziębik A. Life cycle assessment of an integrated oxy-fuel combustion power plant with CO2 capture, transport and storage—Poland case study. Energy. 2015;92(Part 3):328–40. doi:10.1016/j.energy.2015.07.052.
Contreras ML, García-Frutos FJ, Bahillo A. Study of the thermal behaviour of coal/biomass blends during oxy-fuel combustion by thermogravimetric analysis. J Therm Anal Calorim. 2016;123(2):1643–55. doi:10.1007/s10973-015-5067-1.
Normann F, Andersson K, Leckner B, Johnsson F. Emission control of nitrogen oxides in the oxy-fuel process. Prog Energy Combust. 2009;35(5):385–97. doi:10.1016/j.pecs.2009.04.002.
Shao L-M, Fan S-S, Zhang H, Yao Q-S, He P-J. SO2 and NOx emissions from sludge combustion in a CO2/O2 atmosphere. Fuel. 2013;109:178–83. doi:10.1016/j.fuel.2013.01.027.
Wang X, Ren Q, Li L, Li S, Lu Q. TG–MS analysis of nitrogen transformation during combustion of biomass with municipal sewage sludge. J Therm Anal Calorim. 2016;123(3):2061–8. doi:10.1007/s10973-015-4712-z.
Yi B, Zhang L, Huang F, Xia Z, Mao Z, Ding J, et al. Investigating the combustion characteristic temperature of 28 kinds of Chinese coal in oxy-fuel conditions. Energy Convers Manage. 2015;103:439–47. doi:10.1016/j.enconman.2015.06.053.
Abbasi-Atibeh E, Yozgatligil A. A study on the effects of catalysts on pyrolysis and combustion characteristics of Turkish lignite in oxy-fuel conditions. Fuel. 2014;115:841–9. doi:10.1016/j.fuel.2013.01.073.
Ahn S, Choi G, Kim D. The effect of wood biomass blending with pulverized coal on combustion characteristics under oxy-fuel condition. Biomass Bioenergy. 2014;71:144–54. doi:10.1016/j.biombioe.2014.10.014.
López R, Fernández C, Fierro J, Cara J, Martínez O, Sánchez ME. Oxy-combustion of corn, sunflower, rape and microalgae bioresidues and their blends from the perspective of thermogravimetric analysis. Energy. 2014;74:845–54. doi:10.1016/j.energy.2014.07.058.
Zhang Y, Zhang L, Duan F, Jiang X, Sun X, Chyang C. Co-combustion characteristics of sewage sludge with different rank bituminous coals under the O2/CO2 atmosphere. J Therm Anal Calorim. 2015;121(2):729–36. doi:10.1007/s10973-015-4582-4.
Chen J, Mu L, Cai J, Yao P, Song X, Yin H, et al. Pyrolysis and oxy-fuel combustion characteristics and kinetics of petrochemical wastewater sludge using thermogravimetric analysis. Biores Technol. 2015;198:115–23. doi:10.1016/j.biortech.2015.09.011.
Tang Y, Ma X, Lai Z, Fan Y. Thermogravimetric analyses of co-combustion of plastic, rubber, leather in N2/O2 and CO2/O2 atmospheres. Energy. 2015;90(Part 1):1066–74. doi:10.1016/j.energy.2015.08.015.
Zhou Z, Hu X, You Z, Wang Z, Zhou J, Cen K. Oxy-fuel combustion characteristics and kinetic parameters of lignite coal from thermo-gravimetric data. Thermochim Acta. 2013;553:54–9. doi:10.1016/j.tca.2012.11.030.
Folgueras MB, Díaz RM, Xiberta J, Prieto I. Thermogravimetric analysis of the co-combustion of coal and sewage sludge. Fuel. 2003;82(15–17):2051–5. doi:10.1016/S0016-2361(03)00161-3.
Yanfen L, Xiaoqian M. Thermogravimetric analysis of the co-combustion of coal and paper mill sludge. Appl Energy. 2010;87(11):3526–32. doi:10.1016/j.apenergy.2010.05.008.
Kijo-Kleczkowska A, Sroda K, Kosowska-Golachowska M, Musial T, Wolski K. Experimental research of sewage sludge with coal and biomass co-combustion, in pellet form. Waste Manage. 2016;53:165–81. doi:10.1016/j.wasman.2016.04.021.
Werther J, Ogada T. Sewage sludge combustion. Prog Energy Combust. 1999;25(1):55–116. doi:10.1016/S0360-1285(98)00020-3.
García G, Arauzo J, Gonzalo A, Sánchez JL, Ábrego J. Influence of feedstock composition in fluidised bed co-gasification of mixtures of lignite, bituminous coal and sewage sludge. Chem Eng J. 2013;222:345–52. doi:10.1016/j.cej.2013.02.073.
Li XG, Lv Y, Ma BG, Jian SW, Tan HB. Thermogravimetric investigation on co-combustion characteristics of tobacco residue and high-ash anthracite coal. Biores Technol. 2011;102(20):9783–7. doi:10.1016/j.biortech.2011.07.117.
López R, Fernández C, Cara J, Martínez O, Sánchez ME. Differences between combustion and oxy-combustion of corn and corn–rape blend using thermogravimetric analysis. Fuel Process Technol. 2014;128:376–87. doi:10.1016/j.fuproc.2014.07.036.
Moore DS, McCabe GP. Introduction to the practice of statistics. New York: WH Freeman/Times Books/Henry Holt & Co; 1989.
Islam MA, Auta M, Kabir G, Hameed BH. A thermogravimetric analysis of the combustion kinetics of karanja (Pongamia pinnata) fruit hulls char. Bioresour Technol. 2016;200:335–41. doi:10.1016/j.biortech.2015.09.057.
Alvarez A, Pizarro C, Garcia R, Bueno JL, Lavin AG. Determination of kinetic parameters for biomass combustion. Bioresour Technol. 2016;216:36–43. doi:10.1016/j.biortech.2016.05.039.
Niu S, Chen M, Li Y, Xue F. Evaluation on the oxy-fuel combustion behavior of dried sewage sludge. Fuel. 2016;178:129–38. doi:10.1016/j.fuel.2016.03.053.
Magdziarz A, Werle S. Analysis of the combustion and pyrolysis of dried sewage sludge by TGA and MS. Waste Manage. 2014;34(1):174–9. doi:10.1016/j.wasman.2013.10.033.
Yu LY, Li PS. Thermogravimetric analysis of coal and sludge co-combustion with microwave radiation dehydration. J Energy Inst. 2014;87(3):220–6. doi:10.1016/j.joei.2014.03.009.
Otero M, Gomez X, Garcia AI, Moran A. Effects of sewage sludge blending on the coal combustion: a thermogravimetric assessment. Chemosphere. 2007;69(11):1740–50. doi:10.1016/j.chemosphere.2007.05.077.
Yuzbasi NS, Selçuk N. Air and oxy-fuel combustion characteristics of biomass/lignite blends in TGA-FTIR. Fuel Process Technol. 2011;92(5):1101–8. doi:10.1016/j.fuproc.2011.01.005.
Chen C, Lu Z, Ma X, Long J, Peng Y, Hu L, et al. Oxy-fuel combustion characteristics and kinetics of microalgae Chlorella vulgaris by thermogravimetric analysis. Biores Technol. 2013;144:563–71. doi:10.1016/j.biortech.2013.07.011.
Ochoa A, Ibarra Á, Bilbao J, Arandes JM, Castaño P. Assessment of thermogravimetric methods for calculating coke combustion-regeneration kinetics of deactivated catalyst. Chem Eng Sci. 2017;171:459–70. doi:10.1016/j.ces.2017.05.039.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520(1):1–19. doi:10.1016/j.tca.2011.03.034.
Liu X, Chen M, Wei Y. Kinetics based on two-stage scheme for co-combustion of herbaceous biomass and bituminous coal. Fuel. 2015;143:577–85. doi:10.1016/j.fuel.2014.11.085.
Bamford CH, Tipper CFH. Comprehensive chemical kinetics: reactions in the solid state. Amsterdam: Elsevier Scientific Publishing Company; 1980.
Ochoa A, Ibarra Á, Bilbao J, Arandes JM, Castaño P. Assessment of thermogravimetric methods for calculating coke combustion-regeneration kinetics of deactivated catalyst. Chem Eng Sci. 2017;171(Supplement C):459–70. doi:10.1016/j.ces.2017.05.039.
Gil MV, Casal D, Pevida C, Pis JJ, Rubiera F. Thermal behaviour and kinetics of coal/biomass blends during co-combustion. Biores Technol. 2010;101(14):5601–8.
Yorulmaz SY, Atimtay AT. Investigation of combustion kinetics of treated and untreated waste wood samples with thermogravimetric analysis. Fuel Process Technol. 2009;90(7–8):939–46. doi:10.1016/j.fuproc.2009.02.010.
Otero M, Calvo LF, Gil MV, Garcia AI, Moran A. Co-combustion of different sewage sludge and coal: a non-isothermal thermogravimetric kinetic analysis. Bioresour Technol. 2008;99(14):6311–9. doi:10.1016/j.biortech.2007.12.011.
Fang MX, Shen DK, Li YX, Yu CJ, Luo ZY, Cen KF. Kinetic study on pyrolysis and combustion of wood under different oxygen concentrations by using TG-FTIR analysis. J Anal Appl Pyrol. 2006;77(1):22–7. doi:10.1016/j.jaap.2005.12.010.
Acknowledgements
This work was supported by the National Natural Science Foundation of China under No. 51376017.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Niu, S., Chen, M., Li, Y. et al. Co-combustion characteristics of municipal sewage sludge and bituminous coal. J Therm Anal Calorim 131, 1821–1834 (2018). https://doi.org/10.1007/s10973-017-6716-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6716-3