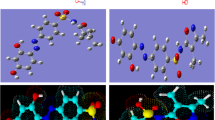
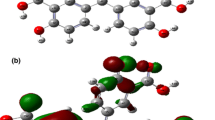
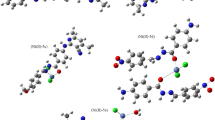
Abstract
A new series of drug complexes from sulfa was prepared using Mn(II), Co(II), Ni(II), Cu(II), Zn(II) and Pt(II) ions. The isolated complexes are deliberately illustrated using spectral, thermal and different theoretical tools. The bidentate mode of coordination is proposed with all complexes. The octahedral configuration is the main structural formula proposed except d8 systems. Significant parameters derived from spectra were deducted to assert on the proposed configurations. XRD and TEM tools display a great conformity in-between for proposing the nano-crystallite sizes of all investigated compounds. Implementing Gaussian 09 program for structural formulas used to obtain the optimized forms. Applying DFT/B3LYP method, the frontier energy gaps were calculated and other important theoretical parameters. Utilizing molecular docking by AutoDock tools used to explain the experimental behavior of organic compounds toward the microorganisms from theoretical visualization. The docked complexes for 4ynu, 4d7h, 1zap, 1ecl, 3e5a, 1y0k and 1bqb protein receptors were investigated and the different energies were calculated. Pt(II), Zn(II) and Cu(II) complexes display significant inhibition for all microorganisms used in biological investigation. Moreover, the IC50 calculated represent the distinguish priority of Ni(II) and Co(II) complexes in overcoming liver cancer.










Similar content being viewed by others
References
Hoffman La Roche Co. Vitamin A the key stone of Animal Nutrition. Swiss Patent No. 416648; 1967.
Sharaby C. Studies of some new cyclodiphosphazane complexes of Fe(III), Fe(II), Co(II), Ni(II), Cu(II), Zn(II) and Cd(II). Synth React Inorg Met Org Chem. 2005;35:133.
Singh RV, Joshi SC, Dwivedi R. Synthetic, magnetic, spectral, antimicrobial and antifertility studies of dioxomolybdenum(VI) unsymmetrical imine complexes having a N–N donor system. Trans. Met. Chem. 2004;29:70–4.
Ferrer S, Borras J, Garcia-Esparia EJ. Complex formation equilibria between the acetazolamide ((5-acetamido-1,3,4-thiadiazole)-2-sulphonamide), a potent inhibitor of carbonicanhydrase, and Zn(II), Co(II), Ni(II) and Cu(II) in aqueous and ethanol-aqueous solutions. Inorg. Biochem. 1990;39:297.
Supuran CT, Minicione F, Scozzafav A, Briganti F, Minicinone G, Ilises MA. Carbonic anhydrase inhibitors—Part 52. Metal complexes of heterocyclic sulfonamides: a new class of strong topical intraocular pressure-lowering agents in rabbits. Eur J Med Chem. 1998;33:247.
Blasco F, Perello L, Latorre J, Borra J, Garcia-Granda SJ. Cobalt(II), Nickel(II), and Copper(II) complexes of sulfanilamide derivatives: synthesis, spectroscopic studies, and antibacterial activity. Crystal structure of [Co(sulfacetamide)2(NCS)2]. Inorg. Biochem. 1996;61:143.
Blasco F, Ortiz R, Perello L, Borras J, Amigo J, Debaerdemaeker TJ. Synthesis and spectroscopy studies of copper(II) nitrate of sulfacetamide drug. Crystal structure of [Cu(sulfacetamide)2(NO3)2]. Antibacterial studies. Inorg. Biochem. 1994;53:117.
Bellú S, Hure E, Trapé M, Rizzotto M, Sutich E, Sigrist M, Moreno V. The interaction between mercury(II) and sulfathiazole. Quim Nova. 2003;26(2):188.
Coombs RR, Ringer MK, Blacquire JM, Smith JC, Neilsen JS. Palladium (II) Schiff base complexes derived from sulfanilamides and aminobenzothiazoles. Trans. Metal Chem. 2005;30:411–8.
El-Baradie K, El-Sharkawy R, El-Ghamry H, Sakai K. Synthesis and characterization of Cu(II), Co(II) and Ni(II) complexes of a number of sulfadrug azodyes and their application for wastewater treatment. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2014;121:180–7.
Mubarak AT, El-Sonbati AZ, Ahmed SM. Supramolecular structural and spectral perspectives of novel ruthenium(III) azodye complexes. J Coord Chem. 2007;60(17):1877–90.
Scott AC. Laboratory control of antimicrobial therapy. In: Gerald J, et al., editors. Practical medical microbiology. 13th ed. Edinburgh: Churchill Livingestone; 1981. p. 161–81.
Mosmann T. Rapid colorimetric assay for cellular growth and survival; application to proliferation and cytotoxicityassays. J Immunol Methods. 1983;65:55–63.
Gangdevi V, Muthumary J. Preliminary studies on cytotoxic effect of fungal taxol on cancer cell lines. Afr J Biotechnol. 2007;6:1382–6.
Wilson AP. Cytotoxicity and viability assays in animal cell culture: a practical approach, 3rd ed. In: John RW editor. Oxford: Oxford University Press; 2000..
Vogel AI. Text book of quantitative inorganic analysis. London: Longman; 1986.
Frisch MJ et al. Gaussian 09, Revision D., Wallingford, CT: Gaussian, Inc.; 2010.
Dennington R II, Keith T, Millam J. GaussView, Version 4.1.2, SemichemInc, Shawnee Mission, KS, 2007.
Halgren TA. Merck molecular force field. I. Basis, form, scope, parametrization, and performance of MMFF94. J Comput Chem. 1998;17(5–6):490–519.
Morris GM, Goodsell DS, et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19(14):1639–62.
Solis DS, Wets RJB. Minimization by random search techniques. Math Oper Res. 1981;6(1):19–30.
Geary W. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. J Coord Chem Rev. 1971;7:81–122.
Nakamoto K, McCarthy PJ. Spectroscopy and structure of metal chelate compounds. New York: Wiley; 1968.
El-Ayaan U, Youssef MM, Al-Shihry S. Mn(II), Co(II), Zn(II), Fe(III) and U (VI) complexes of 2-acetylpyridine 4 N-(2-pyridyl) thiosemicarbazone (HAPT); structural, spectroscopic and biological. J Mol Struct. 2009;936:213–9.
Shah RK, Abou-Melha KS, Saad FA, Yousef T, Al-Hazmi GAA, Elghalban MG, Khedr AM, El-Metwaly NM. Elaborated studies on nano-sized homo-binuclear Mn(II), Fe(III), Co(II), Ni(II), and Cu(II) complexes derived from N2O2 Schiff base, thermal, molecular modeling, drug-likeness, and spectral. J Therm Anal Calorim. 2015. doi:10.1007/s10973-015-4838-z.
Ferrari MB, Capacchi S, Reffo G, Aelosi G, Tarasconi P, Albertini R, Pinellis S, Lunghi P. Synthesis, structural characterization and biological activity of P-fluorobenzaldehyde thiosemicarbazones and of a nickel complexes. J Inorg Biochem. 2000;81:89.
Jorgensen CK. Fractional charges, integral oxidation states and the nephelauxetic effect in the five transition groups. Helv Chim Acta. 1967;50:131–46.
Deplano P, Pilia L, Espa D, Mercuri ML, Serpe A. Square-planar d 8 metal mixed-ligand dithiolene complexes as second order nonlinear optical chromophores: structure/property relationship. Coord Chem Rev. 2010;254:1434–47.
Lever ABP. Inorganic electronic spectroscopy. Amsterdam: Elsevier; 1986.
Soleimani E. Synthesis, characterization and anti-microbial activity of a novel macrocyclic ligand derived from the reaction of 2,6-pyridinedicarboxylic acid with homopiperazine and its Co(II), Ni(II), Cu(II), and Zn(II) complexes. J Mol Struct. 2011;995:1–8.
Sanderson RT. Inorganic chemistry, Chap. 6. New York: Reinhold; 1967.
Stoklosa H. Computer program for calculation of charge distributions in molecules. J Chem Educ. 1973;50(4):290.
Park HI, Ming LJ. The mechanistic role of the coordinated tyrosine in astacin. J Inorg Biochem. 1998;72:57.
Hathaway BJ, Billing DE. The electronic properties and stereochemistry of mono-nuclear complexes of the copper(II) ion. Coord Chem Rev. 1970;5:143.
Hathaway BJ. A new look at the stereochemistry and electronic properties of complexes of the copper(II) ion. Struct Bond. (Berlin). 1984;57:55.
Montgomery H, Lingefetter EC. The crystal structure of Tutton’s salts. IV. Cadmium ammonium sulfate hexahydrate. Acta Cryst. 1966;20: 728.
Kivelson D, Neiman RJ. ESR studies on the bonding in copper complexes. Chem Phys. 1961;35:149.
Maurya RC, Rajput S. Oxovanadium(IV) complexes of bioinorganic and medicinal relevance: synthesis, characterization, and 3D molecular modeling and analysis of some oxovanadium(IV) complexes involving O, O-donor environment. J Mol Struct. 2004;687:35.
Wellman JA, Hulsbergen FB, Verbiest J, Reedijk J. Influence of alkyl chain length in N-alkyl imidazoles upon the complex formation with transition-metal salts”. J Inorg Nucl Chem. 1978;40:143.
Yokoi H, Addison AW. Spectroscopic and redox properties of pseudotetrahedral copper(II) complexes. Their relation to copper proteins. Inorg Chem. 1977;16:1341.
Ray RK, Kauffman GR. EPR Spectra and covalency of bis(amidinourea/O-alkyl-1-amidinourea)copper(II) complexes Part II. Properties of the CuN4 2− chromophore. Inorg Chem Acta. 1990; 173: 207.
Chikate RC, Padhye SB. Transition metal quinone–thiosemicarbazone complexes 2: magnetism, ESR and redox behavior of iron (II), iron (III), cobalt (II) and copper (II) complexes of 2-thiosemicarbazido-1,4-naphthoquinone. Polyhedron 2005; 24:1689.
Cullity BD. Elements of X-ray diffraction. 2nd ed. Reading, MA: Addison-Wesley Inc.; 1993.
Fahem AA. Comparative studies of mononuclear Ni(II) and UO2(II) complexes having bifunctional coordinated groups: synthesis, thermal analysis, X-ray diffraction, surface morphology studies and biological evaluation. Spectrochim. Acta A. 2012;88:10–22.
Shahrjerdi A, Davarani SSH, Najafi E, Amini MM. Sonoelectrochemical synthesis of a new nano lead(II) complex with quinoline-2-carboxylic acid ligand: a precursor to produce pure phase nano-sized lead(II) oxide. Ultrason Sonochem. 2015;22:382–90.
Yamanuchi T, Tsukahava Y, Yamada K, Sakata T, Wada Y. Nucleation and growth of magnetic Ni–Co (core–shell) nanoparticles in a one-pot reaction under microwave irradiation. Chem Mater. 2011;23:75–84.
Ritch JS, Chivers T, Ahmad K, Afzaal M, Brien PO. Synthesis, structures, and multinuclear NMR spectra of Tin(II) and Lead(II) complexes of tellurium-containing imidodiphosphinate ligands: preparation of two morphologies of phase-pure PbTe from a single-source precursor. Inorg Chem. 2010;49:1198.
Mokari T, Zhang M, Yang P. Shape, size, and assembly control of PbTe nanocrystals. J Am Chem Soc. 2007;129:9864–5.
Urban JJ, Talapin DV, Shevchenko EV, Murray CB. Self-assembly of PbTe quantum dots into nanocrystal superlattices and glassy films. J Am Chem Soc. 2006;128:3248–55.
Zhang B, He J, Tritt TM. Size-selective high-yield growth of lead telluride (PbTe) nanocrystals using a chemical vapor deposition technique. Appl Phys Lett. 2006;88:043119.
Fleming I. Frontier orbitals and organic chemical reactions. London: Wiley; 1976.
Ray RK, Kauffman GR. EPR spectra and covalency of bis(amidinourea/o-alkyl-1-amidinourea)copper (II) complexes. Part II. Properties of the CuN 42—chromophore. Inorg Chem Acta. 1990; 173: 207–14.
Chikate RC, Padhye SB. Transition metal quinone-thiosemicarbazone complexes 2: magnetism, ESR and redox behavior of iron (II), iron (III), cobalt (II) and copper (II) complexes of 2-thiosemicarbazido-1,4-naphthoquinone. Polyhedron 2005; 24:1689–700.
SagdincS KöksoyB, KandemirliF BayariSH. Theoretical and spectroscopic studies of 5-fluoro-isatin-3-(N-benzylthiosemicarbazone) and its zinc(II) complex. J Mol Struct. 2009;917:63–70.
Tripathi SK, Muttineni R, Singh SK. Extra precision docking, free energy calculation and molecular dynamics simulation studies of CDK2 inhibitors. J Theor Biol. 2013;334:87–100.
Ballhausen CJ. Intensities of spectral bands in transition metal complexes. Prog Inorg Chem. 1960;2:251–265.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saad, F.A. Elaborated molecular docking and DFT/B3LYP studies for novel sulfa drug complexes, spectral and antitumor investigations. J Therm Anal Calorim 129, 425–440 (2017). https://doi.org/10.1007/s10973-016-6017-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-6017-2