Abstract
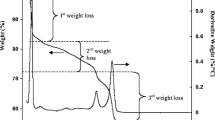
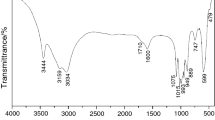
The thermodynamic properties of the fabrication of NiTiO3 material in different reaction times are reported. The design of this material is accessible through a new efficient sol–gel methods, utilizing Ni(Ac)2·4H2O and Ti(OiPr)4 as starting materials for the formation of NiTiO3 final product through thermal decomposition. The thermogravimetric (TG) and differential scanning calorimetric (DSC) techniques were used to analyze the reaction of Ni(Ac)2·4H2O and Ti(OiPr)4, which produces precursor materials at 0.5, 1, 2, 24, 48 and 72 h of reaction times, as well as the thermal stability of these precursors and the final product. The DSC data show an exothermic phenomenon of releasing large amount of energy of −1393 J/g at T Peak 655 K due to the first event of decomposition started at T Onset 607 K and finished at T Endset 663 K for the precursor materials obtained at 0.5 h of reaction, showing the presence of starting materials in this precursor. A similar exothermic behavior was observed in the sample of 1 h of reaction time and was vanished in the materials obtained at 2–72 h of reaction, indicating the influence of the time on the completion of reaction and formation of NiTiO3 crystalline phase as final product of thermal decomposition. In addition, using the information obtained from the TG/DSC, XRD and FTIR analyses, the optimum temperature for the thermal decomposition of the precursor materials to NiTiO3 with fairly high crystallinity was also determined and discussed.








Similar content being viewed by others
References
Yamamoto O, Takeda Y, Kanno R, Noda M. Perovskite-type oxides as oxygen electrodes for high temperature oxide fuel cells. Solid State Ion. 1987;22:241–6.
Navrotsky A. Energetics and crystal chemical systematics among ilmenite, litium niobate and perovskite structures. Chem Mater. 1998;10:2787–93.
Giaquinta DM. Loye Zur H-C. Structural predictions in the ABO3 phase diagram. Chem Mater. 1994;6:365–72.
Neil JM, Navrotsky A, Kleppa O. The enthalpy of ilmenite-perovskite transformation in cadmium titanate. J Inorg Chem. 1971;10:2076–7.
Bedoya-Hincapié CM, Restrepo-Parra E, Olaya-Flórez JJ, Alfonso JE, Flores-Ruiz FJ, Espinoza-Beltrán FJ. Ferroelectric behavior of bismuth titanate thin films grown via magnetron sputtering. Ceram Int. 2014;40:11831–6.
Liu XC, Hong R, Tian C. Tolerance factor and the stability discussion of ABO3-type ilmenite. J Mater Sci Mater Electron. 2009;20:323–7.
Salvador P, Gutiérrez C, Goodenough JB. Photoelectrochemical properties of n-type NiTiO3. J Appl Phys. 1982;53(10):7003–13.
Mohammadi MR, Fray DJ. Mesoporous and nanocrystalline sol–gel derived NiTiO3 at the low temperature: controlling the structure, size and surface area by Ni: Ti molar ratio. Solid State Sci. 2010;12:1629–40.
Lin YJ, Chang YH, Yang WD, Tsai BS. Synthesis and characterization of ilmenite NiTiO3 and CoTiO3 prepared by a modified Pechini method. J Non-Cryst Solid. 2006;352:789–94.
Renzhi M, Bando Y, Sasaki T. Nanotubes of lepidocrocite titanates. Chem Phys Lett. 2003;380:577–82.
Qu Y, Zhou W, Ren Z, Du S, Meng X, Tian G, Pan K, Wang G, Fu H. Facile preparation of porous NiTiO3 nanorods with enhanced visible-light-driven photocatalytic performance. J Mater Chem. 2012;22:16471–6.
Diallo PT, Boutinaud P, Mahiou R, Cousseins JC. Red Luminescence in Pr3+-Doped Calcium Titanates. Phys Status Solidi (a). 1997;160:253–63.
Shih S-J, Tzeng W-L. Manipulation of morphology of strontium titanate particles by spray pyrolysis. Powder Technol. 2014;264:291–7.
Nguyen-Phan T-D, Nguyen-Huy C, Shin EW. Morphological evolution of hierarchical nickel titanates by elevation of the solvothermal temperature. Mater Lett. 2014;131:217–21.
Moghiminia S, Farsi H, Raissi H. Comparative optical and electrochemical studies of nanostructured NiTiO3 and NiTiO3-TiO2 prepared by a low temperature modified sol–gel route. Electrochim Acta. 2014;132:512–23.
Ruiz-Preciado MA, Kassiba A, Gibaud A, Morales-Acevedo A. Comparision of nickel titanate (NiTiO3) powders synthesized by sol–gel and solid state reaction. Mater Sci Semicond Process. 2015;37:171–8.
Lopes KP, Cavalcante LS, Simôes AZ, Varela JA, Longo E, Leite ER. NiTiO3 powders obtained by polymeric precursor method: synthesis and characterization. J Alloys Compd. 2009;468:327–32.
Perrin FX, Nguyen V, Vernet JL. FT-IR spectroscopy of acid-modified titanium alkoxides: investigations on the nature of carboxylate coordination and degree of complexation. J Sol–Gel Technol. 2003;28:205–15.
The International Centre for Diffraction Data. ICDD/PDF 33-0960. 2014.
Busca G, Ramis G, Amores JMG, Escribano VS, Piaggio P. FT Raman and FTIR studies of titanias and metatitanate powders. J Chem Soc Faraday Trans. 1994;90:3181–90.
Baraton MI, Busca G, Prieto MC, Ricchiardi G, Escribano VS. On the vibrational spectra and structure of FeCrO3 and of the ilmenite-type compounds CoTiO3 and NiTiO3. J Solid State Chem. 1994;112:9–14.
Mironova-Ulmane N, Kuzmin A, Sildos I, Pärs M. Polarisation dependent raman study of single-crystal nickel oxide. Cent Eur J Phys. 2011;9:1096–9.
Lucena PR, Pessoa-Neto OD, Santos IMG, Souza AG, Longo E, Varela JA. Synthesis by the polymeric precursor method and characterization of undoped and Sn, Cr and V-doped ZrTiO4. J Alloys Compd. 2005;397:255–9.
Leite ER, Varela JA, Longo E, Paskocimas CA. Influence of polymerization on the synthesis of SrTiO3: part II. Particle and agglomerate morphologies. Ceram Int. 1995;21:153–8.
Santos IMG, Longo E, Varela JA, Leite ER. Sintering of tin oxide processed by slip casting. J Eur Ceram Soc. 2000;20:2407–13.
Acknowledgements
Laboratory of Thermal Analysis Prof. Ivo Giolito (Latig–USP) and the Laboratory of the f Block Elements (LEB-f) to measures of TG/DSC, Núcleo de Petróleo (Labpetro—UFES) to measures of FTIR and funding agency Coordenação de Apoio a Amparo a Pesquisa (CAPES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigues, R.V., Muri, E.J.B., da Cruz, P.C.M. et al. Thermogravimetric study on preparation of NiTiO3 in different reaction times. J Therm Anal Calorim 126, 1499–1505 (2016). https://doi.org/10.1007/s10973-016-5836-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5836-5