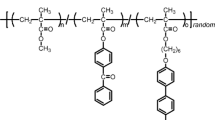
Abstract
Two homologous series of triblock molecules of the H(CH2)n(CF2)6(CH2)nH and F(CF2)n(CH2)6(CF2)nF general formulas with n = 6, 8, 10, and 12 have been synthesized and investigated using two complementary methods: differential scanning calorimetry and polarized optical microscopy in order to investigate their thermal behavior, establish phase diagram, and calculate thermodynamic parameters of the observed phase transitions. Although different from commonly known liquid crystal materials, all the compounds studied form thermotropic liquid crystalline phases (smectic or nematic) and undergo vitrification process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the classic approach, there are two main groups of molecules capable of forming liquid crystalline (LC) phases, i.e., anisometric and amphiphilic molecules. The former ones, of either rod- or disk-like shape, show thermotropic mesophases [1, 2]. On the other hand, amphiphilic molecules, built of two segments: polar and apolar, are known for forming lyotropic liquid crystalline mesophases. Semifluorinated alkanes (SFAs), consisting of hydrogenated and perfluorinated segments covalently bound within one molecule, are non-typical as regards conventional definition of liquid crystalline materials. Namely, although purely hydrophobic and rod-like shaped, their structure is not typically mesogenic since most of the mesogenic molecules have a rigid aromatic/cyclic frame (e.g., biphenyl, azobenzene, cyclopentanoperhydrophenanthrene) or multiple bonds extended in distal positions by long-chained alkyl substituents. Instead, SFAs possess a rigid fluorinated segment of helical conformation attached to flexible, zig-zag alkyl chains. Interestingly, although SFAs do not possess any polar group in their structure, they are amphiphilic and behave like surfactants as proved in Refs. [3, 4]. The simplest molecules of this kind are diblock semifluorinated alkanes of the F(CF2)m(CH2)nH formula (in short FmHn). First molecule of this kind, for which liquid crystalline phase was observed, was F10H10 [5]. Further investigations revealed that—for majority of the investigated diblocks—the LC phase was classified as smectic B (see Refs. [6–8]), although for some homologs higher ordering (smectic G or J) was also identified.
Recently, we have synthesized a series of triblock SFAs of the H(CH2)n(CF2)6(CH2)nH general formula (in short HnF6Hn) with n = 12, 14, 16, 18, and 20, which were found to exhibit thermotropic LC phases [9]. In this paper, we have extended our study to the HnF6Hn-type triblocks with shorter alkyl segments (n = 6, 8, and 10). The next homolog of this kind, i.e., with n = 12, has already been investigated; however, for the sake of comparison, the results adopted from Ref. [9] are also shown here. Additionally, we have synthesized and investigated triblocks of different structures, i.e., with an alkyl fragment extended in distal positions by two fluorinated segments, i.e., F(CF2)n(CH2)6(CF2)nF (in short FnH6Fn) with n = 6, 8, 10, and 12, and performed a comparative study of both kinds of triblocks.
Experimental
Materials
Synthesis of semifluorinated triblock molecules
Homologs from FnH6Fn and HnF6Hn series were synthesized following experimental conditions, which were previously described by Twieg and Rabolt [10] (for FmHnFm compounds) as well as applied in our former paper [9] (for HnFmHn compounds). Composition and purity of the synthesized triblocks were confirmed by elemental analysis. Additionally, nuclear magnetic resonance spectra were recorded for HnF6Hn compounds, which were soluble in standard laboratory solvents in contrast to FmH6Fm series. Analytical data of the synthesized compounds are as follows:
1,1,1,2,2,3,3,4,4,5,5,6,6,13,13,14,14,15,15,16,16,17,17,18,18,18-hexacosafluorooctadecane (F 6 H 6 F 6 ): composition measured: C 30.14 % H 1.89 % (calculated: C 29.93 % H 1.67 % F 68.39 %);
1,1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,15,15,16,16,17,17,18,18,19,19,20,20,21,21,22,22,22-tetratriacontafluorodocosane (F 8 H 6 F 8 ): composition measured: C 28.75 % H 1.38 % (calculated: C 28.65 % H 1.31 % F 70.04 %);
1,1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,17,17,18,18,19,19,20,20,21,21,22,22,23,23,24,24,25,25,26,26,26-dotetracontafluorohexacosane (F 10 H 6 F 10 ): composition measured: C 28.00 % H 1.11 % (calculated: C 27.82 % H 1.08 % F 71.10 %);
1,1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,19,19,20,20,21,21,22,22,23,23,24,24,25,25,26,26,27,27,28,28,29,29,30,30,30-pentacontafluorotriacontane (F 12 H 6 F 12 ): composition measured: C 26.94 % H 0.97 % (calculated: C 27.25 % H 0.91 % F 71.84 %);
7,7,8,8,9,9,10,10,11,11,12,12-dodecafluorooctadecane (H 6 F 6 H 6 ): 1H NMR (300 MHz, CDCl3) δ 2.16–1.93 (m, 4H), 1.66–1.53 (m, 4H), 1.45–1.20 (m, 20H), 0.89 (t, J = 6.8 Hz, 6H); composition measured: C 45.81 % H 5.50 % (calculated: C 45.96 % H 5.57 % F 48.47 %);
9,9,10,10,11,11,12,12,13,13,14,14-dodecafluorodocosane (H 8 F 6 H 8 ): 1H NMR (300 MHz, CDCl3) δ 2.16–1.93 (m, 4H), 1.67–1.54 (m, 4H), 1.46–1.25 (m, 12H), 0.90 (t, J = 6.8 Hz, 6H); composition measured: C 49.90 % H 6.45 % (calculated: C 50.19 % H 6.51 % F 43.30 %)
11,11,12,12,13,13,14,14,15,15,16,16-dodecafluorohexacosane (H 10 F 6 H 10 ): 1H NMR (300 MHz, CDCl3) δ 2.16–1.92 (m, 4H), 1.66–1.50 (m, 4H), 1.42–1.24 (m, 28H), 0.89 (t, J = 6.6 Hz, 6H); composition measured: C 53.44 % H 7.17 % (calculated: C 53.60 % H 7.27 % F 39.13 %);
13,13,14,14,15,15,16,16,17,17,18,18-dodecafluorotriacontane (H 12 F 6 H 12 ): 1H NMR (300 MHz, CDCl3) δ 2.16–1.93 (m, 4H), 1.67–1.52 (m, 4H), 1.44–1.20 (m, 36H), 0.88 (t, J = 6.7 Hz, 6H); composition measured: C 56.79 % H 7.84 % (calculated: C 56.41 % H 7.89 % F 35.69 %).
Methods
Differential scanning calorimetry
DSC measurements were performed for the two series of triblocks, HnF6Hn and FnH6Fn, using Mettler-Toledo 821e and 822e calorimeters in the temperature range of 150–410 K with the scanning rates of 10, 5, and 2 K min−1. The masses of the HnF6Hn bulk samples with n = 6, 8, 10, and 12 were equal to 13.07, 4.35, 2.76, and 6.93 mg, respectively. The masses of the FnH6Fn bulk samples with n = 6, 8, 10, and 12 were equal to 6.78, 3.93, 3.34, and 4.19 mg, respectively. The samples were placed in hermetically sealed aluminum pans (30 μL). The instrument was calibrated using the literature data for indium and ice melting points. The enthalpy changes (ΔH) linked up with observed transitions were calculated by numerical integration of the DSC curves under the peaks of the anomalies. The estimations of entropy changes (ΔS) were calculated using the following formula: ΔS = ΔH/T C. The transition temperatures T C were considered to be the peak temperatures (T peak) from the DSC curves obtained on heating and cooling. The transition temperatures are given with accuracy of ±0.5 K. The values of the enthalpy and entropy changes at the transitions are given with the accuracy of about 20 %.
Polarized optical microscopy
The textures of different phases observed were identified using Biolar PI polarized microscope (PZO Warsaw). The temperature was stabilized by Linkam THM 600 silver heating/cooling stage and TMS 90 temperature controller. Transition temperatures were measured by platinum resistance thermometer stabilized with temperature accuracy of ±0.1 K. The observations were carried out both during heating and cooling in the temperature range of 170–420 K.
Results and discussion
Studies of polymorphism of all the investigated homologous substances of two types of semifluorinated triblock molecules were carried out using two complementary experimental methods: differential scanning calorimetry (DSC) and texture observations with the polarized optical microscopy (POM). Results of the performed measurements let us verify if the investigated compounds form ordered solid, plastic, liquid crystalline or vitreous phases and characterize them by estimation of thermodynamic parameters, such as transition temperature, enthalpy, and entropy changes at the transitions. All the DSC curves were obtained for virgin samples, both during heating and subsequent cooling, and registered with a scanning rate of 10 K min−1. For clarification of some of our results, we have performed additional DSC measurements with smaller scanning rates (5 or 2 K min−1). Also, all the images of textures shown herein were recorded for bulk samples with the rates of 10 or 5 K min−1.
HnF6Hn homologs
The H6F6H6 compound is the only one investigated in this work which is isotropic liquid at room temperature. Based on POM observations, it undergoes three phase transitions. Two of them, observed consecutively in the DSC curve during heating at T C2 = 200.5 K and T C1 = 242.1 K, occur in the solid state. The first one at T C2 is connected with the transition between glass state and LC smectic phase (GSmX → SmX). From texture observation, we could not establish what type of smectic phase it is. However, due to the anticipated theoretical sequence of LC thermotropic mesophases, it can be either smectic K or smectic H phase. The second transition at T C1 occurs between two LC smectic phases (SmX → SmE). The third transition at T Iso = 294.2 K is associated with conversion into isotropic liquid (SmE → Iso). Interestingly, there is also one small and broad anomaly with a maximum observed at 274 K (on heating) and 270 K (on cooling) in both DSC curves (see Fig. 1). However, the existence of additional phase corresponding to this anomaly in this temperature region was not confirmed by optical microscopy. Images of the observed phases are presented in Fig. 2.
Taking into consideration the results obtained using the two methods, the following phase sequence could be established: \({\text{Iso}}\mathop{\longrightarrow}\limits^{286K}{\text{SmE}}\mathop{\longrightarrow}\limits^{239K}{\text{SmX}}\mathop{\longrightarrow}\limits^{181K}{\text{GSmX}}\).
Figure 3 presents the DSC curves obtained for the H8F6H8 homolog together with POM images of smectic E (SmE) phase with characteristic mosaic texture and glass of smectic E (GSmE) phase. There are four anomalies visible both during heating and cooling. Phase identification based on texture observation let us determine that the high-temperature large and sharp anomaly observed during heating at T Iso = 318.5 K is connected with isotropization process, whereas the other three low-temperature anomalies visible in the DSC curves below 290 K are connected with three-step transition between LC smectic phase (SmE) and glass phase (SmE ↔ GSmE). The polarized optical microscopy observation of the steadily cooled sample complemented with DSC results led to the following phase sequence: \({\text{Iso}}\mathop{\longrightarrow}\limits^{314K}{\text{SmE}}\mathop{\longrightarrow}\limits^{265K,249K,227K}{\text{GSmE}}\)
The DSC results obtained for the H10F6H10 homolog are presented in Fig. 4. Two endothermic anomalies can be distinguished in the DSC curve registered during heating with a scanning rate of 10 K min−1, one small with a maximum at T C2 = 294.8 K and another large and very broad with a maximum at T C1 = 319.9 K. Based on POM observation, it could be established that the first anomaly is connected with the transition between two plastic crystal phases (PCrII → PCrI) and the second anomaly is connected with the transition between plastic crystal and nematic phases (PCrI → N). The isotropization process can be visible in the DSC curve after applying smaller scanning rate (see DSC curve obtained upon heating with 2 K min−1 in Fig. 4). The Iso → N and N → PCrI transitions are clearly visible in the DSC curves registered upon cooling with both scanning rates. In turn, the transition from PCrI to PCrII is poorly stressed in the DSC curve during cooling—small peak at 296 K can be connected with this transition. The transition from PCrI to PCrII was not observed with optical microscopy. Textures of both plastic crystal phases and nematic phase observed upon heating are presented in Fig. 5. Additionally, very broad and small anomalies can be also visible after magnification at about 220 and 179 K during heating, and at about 263, 211, and 175 K during cooling in the DSC curves (see inset to Fig. 4). This is most probably connected with gradual vitrification of plastic crystal phase (PCrII). However, the cracks on the plastic crystal texture were not observed. Taking into consideration the DSC and POM results, the following phase sequence can be established: \({\text{Iso}}\mathop{\longrightarrow}\limits^{328K}{\text{N}}\mathop{\longrightarrow}\limits^{316K}{\text{PCrI}}\mathop{\longrightarrow}\limits^{296K}{\text{PCrII}}\mathop{\longrightarrow}\limits^{263K,211K,175K}{\text{GPCrII}}\).
Figure 6 presents the DSC diagram obtained for the last homolog investigated in this group, i.e., H12F6H12. The DSC and POM results have been already presented in our previous paper [9]. The compound exhibits two LC phases, smectic B and E, and one crystal phase, which gradually undergoes vitrification process below 300 K.
DSC curves of H12F6H12 presented between 230 and 345 K registered with a scanning rate of 10 K min−1 [9]
FnH6Fn homologs
The DSC curves obtained for F6H6F6 are presented in Fig. 7. In temperatures between 240 and 340 K, one large and sharp anomaly can be observed during heating with a maximum at T Iso = 326 K and one large exothermic anomaly with a maximum at T cryst = 319 K with a shoulder visible at about 314.5 K during cooling process. Based on the texture observation, the anomalies observed during heating and cooling are connected with conversion of the LC smectic B (SmB) phase into isotropic liquid and crystallization process, respectively. Although there is an exothermic shoulder visible in the DSC curve during cooling, upon further cooling the image of texture did not change, even below 220 K. This behavior suggests that glass of SmB phase is formed in low temperatures. The temperature of the transition to the glass phase of SmB was not possible to be established from POM measurements, as cracks on the texture were not observed. Except for high-temperature anomaly, the DSC curve obtained during cooling shows two deflections at about 221 and 162 K, which indicates—most probably—a gradual vitrification process. During subsequent heating, two deflections are visible at about 164 and 223 K in the DSC curve (see inset of Fig. 7).
Based on the discussed results, the phase sequence is as follows: \({\text{Iso}}\mathop{\longrightarrow}\limits^{319K}{\text{SmB}}\mathop{\longrightarrow}\limits^{221K,162K}{\text{GSmB}}\).
The F8H6F8 homolog also exhibits one mesophase, smectic B (SmB) phase, and one plastic crystal phase. Based on the optical microscopy observation of the steadily cooled sample and DSC results, the following phase sequence occurs: \({\text{Iso}}\mathop{\longrightarrow}\limits^{353K}{\text{SmB}}\mathop{\longrightarrow}\limits^{348K}{\text{PCr}}\mathop{\longrightarrow}\limits^{211K}{\text{GPCr}}\).
During cooling of the sample from isotropic liquid (Iso), the Iso → SmB phase transition is observed in DSC curve at 353 K (see Fig. 8). The characteristic mosaic texture of SmB phase was registered for this compound at 349 K, which is shown in Fig. 9a. The rate of the SmB–PCr transition is very fast. During cooling, this transition occurs in the DSC curve at T C1 = 348 K. During heating, only the SmB → Iso transition is observed in the DSC curve at T Iso = 356 K, even for larger scanning rates applied. One plastic crystal phase was discovered by the microscopy observations as no other textures were revealed. The conversion to glass phase was manifested by rapid appearance of cracks in the PCr texture at 212 K. The cracks disappeared at 278 K. The results of texture observations and DSC are in a good agreement. The transition into glass phase PCr → GPCr is visible in the DSC curve at 211 K (blue lower curve in the inset to Fig. 8), and the transition into plastic crystal phase GPCr → PCr appeared at 236 and 254 K (red upper curve in the inset of Fig. 8).
Figure 10 presents DSC curves obtained for F10H6F10 between 270 and 390 K. The DSC and POM results have been already presented in our previous paper [11]. The investigated compound exhibits at least four different phases in the following sequence during cooling procedure: \({\text{Iso}}\mathop{\longrightarrow}\limits^{368K}{\text{SmA}}\mathop{\longrightarrow}\limits^{339K}{\text{PCr}}\mathop{\longrightarrow}\limits^{320K}{\text{GPCr}}\). DSC results suggest existence of one more distinguishable phase between 339 and 329 K (upon cooling), but it was not confirmed by optical microscopy—images of the textures recorded between 339 and 321 K were the same.
DSC curves of F10H6F10 presented between 270 and 390 K registered with a scanning rate of 10 K min−1 together with texture of SmA phase (T = 361 K) [11]
The DSC curves obtained for F12H6F12 between 290 and 410 K and corresponding textures of the observed phases are presented in Figs. 11 and 12, respectively. The temperatures obtained with POM method are shifted significantly toward higher temperatures as compared to the DSC results. During heating, the transitions GPCr → PCr, PCr → SmA, and SmA → Iso were observed at 364, 409, and 412 K, respectively. In turn, during cooling, the transitions Iso → SmA, SmA → PCr, and PCr → GPCr were observed at 400, 397, and 334 K, respectively. Interestingly, during heating with a rate of 5 K min−1, the crystal nucleation appeared in the GPCr texture at 240 K visible as small spots in the texture in Fig. 12d. These spots keep growing till 250 K (see Fig. 12e) and then melt. With a rate higher than 5 K min−1, this phenomenon is not observed as the crystallization rate is low. The phase sequence in this compound: \({\text{Iso}}\mathop{\longrightarrow}\limits^{391K}{\text{SmA}}\mathop{\longrightarrow}\limits^{366K}{\text{PCr}}\mathop{\longrightarrow}\limits^{326K,316K}{\text{GPCr}}\) is basically the same as that observed for the F10H6F10 homolog, except for the transitions appearing at higher temperature.
Figure 13 summarizes the calorimetric results obtained during heating, while Fig. 14 shows glass and isotropization temperatures for both series of the investigated homologs, HnF6Hn and FnH6Fn. The thermodynamic parameters of the detected phase transitions, such as transition temperatures T C and enthalpy and entropy changes, ΔH and ΔS, based on the DSC results, are compiled in Table 1.
Conclusions
Two series of triblock molecules consisting of hydrogenated and perfluorinated building blocks, located either in the middle or at the end of molecules, differing in the length of terminal segments have been synthesized. The performed experiments, involving DSC and POM, allowed us to draw the following conclusions:
-
All the investigated HnF6Hn and FnH6Fn triblock molecules form thermotropic liquid crystalline phases (smectic or nematic). One LC phase of smectic ordering (SmA or SmB) was observed for all the studied four homologs of the FnH6Fn type. Among the HnF6Hn homologs, one smectic (SmE) phase and one nematic phase were observed in H8F6H8 and H10F6H10, respectively. The other two compounds in this series, i.e., H6F6H6 and H12F6H12, exhibit two smectic phases.
-
For both HnF6Hn and FnH6Fn series, it can be observed that the longer hydrocarbon or perfluorinated segments extended in distal positions, the higher temperature of the transition into isotropic liquid (Sm or N → Iso). Isotropization temperatures observed for the FnH6Fn series are significantly higher than those for HnF6Hn.
-
All the studied compounds from both series (HnF6Hn and FnH6Fn) undergo vitrification process, which—for the majority of the homologs under investigation—proceeds gradually. Generally, the glass temperature is higher for compounds with longer hydrocarbon or fluorinated segments extended in distal positions.
-
One crystalline phase, most probably plastic one, has been observed in all the compounds from FnH6Fn series, except for F6H6F6. Among the HnF6Hn homologs studied in this work, only H10F6H10 exhibited plastic crystal phases.
-
No metastable behavior was observed in any of the studied compounds.
-
Analyzing the results obtained for the previously studied compounds of the HnF6Hn type with even number of n = 12, 14, 16, 18, and 20 [9], it can be concluded that molecules with n between 20 and 12 exhibit two LC phases, SmE and SmB (except for H14F6H14 where three LC phases were observed: SmE, SmB, and SmA). The HnF6Hn compounds with n = 10 and 8, namely H10F6H10 and H8F6H8, show one LC phase, nematic and SmE, respectively. Interestingly, the homolog with the shortest hydrocarbon segments extended in distal positions studied so far—H6F6H6—exhibits two LC smectic phases.
References
Demus D, Goodby JW, Gray GW, Spiess HW, Vill V. Handbook of liquid crystals. Weinheim: Wiley-VCH; 1998.
Ramamoorthy A, editor. Thermotropic liquid crystals: recent Advances. New York: Springer; 2007.
Turberg MP, Brady JE. Semifluorinated hydrocarbons: primitive surfactant molecules. J Am Chem Soc. 1988;110:7797–801.
Gaines GL. Surface activity of semifluorinated alkanes: F(CF2)m(CH2)nH. Langmuir. 1991;7:3054–6.
Mahler W, Guillon D, Skoulios A. Smectic liquid crystal from (perfluorodecyl)decane. Mol Cryst Liq Cryst Lett. 1985;2:111–9.
Broniatowski M, Dynarowicz-Łątka P, Witko W. Critical influence of the alkane length in surface and liquid-crystalline properties of perfluorodecyl-n-alkanes. J Fluor Chem. 2005;126:79–86.
Krafft MP, Riess JG. Chemistry, physical chemistry, and uses of molecular fluorocarbon-hydrocarbon diblocks, triblocks, and related compounds—unique “apolar” components for self-assembled colloid and interface engineering. Chem Rev. 2009;109:1714–92.
Tschierske C, editor. Liquid crystals: materials design and assembly. Topics in current chemistry, vol. 318. New York: Springer; 2012. p. 1–109.
Chachaj-Brekiesz A, Górska N, Osiecka N, Mikuli E, Dynarowicz-Łątka P. Synthesis and thermal behaviour of triblock semifluorinated n-alkanes. J Therm Anal Calorim. 2016;124:251–60.
Twieg RJ, Rabolt JF. Structural studies of semifluorinated n-alkanes. 3. Synthesis and characterization of F(CF2)n(CH2)m(CF2)nF. Macromolecules. 1988;21:1806–11.
Chachaj-Brekiesz A, Górska N, Osiecka N, Makyła-Juzak K, Dynarowicz-Łątka P. Surface and liquid-crystalline properties of FmHnFm triblock semifluorinated n-alkanes. Mater Sci Eng C. 2016;62:870–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chachaj-Brekiesz, A., Górska, N., Osiecka, N. et al. Mesophases of non-conventional liquid crystalline molecules. J Therm Anal Calorim 126, 689–697 (2016). https://doi.org/10.1007/s10973-016-5511-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5511-x