Abstract
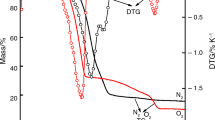
The formation of light rare earth metal oxides such as CeO2, La2O3, Sm2O3, Nd2O3 and Pr2O3 from thermal decomposition of its nitrate precursors (Ce(NO3)3·6H2O, La(NO3)3·6H2O, Sm(NO3)3·6H2O, Nd(NO3)3·6H2O and Pr(NO3)3·6H2O) have been investigated by thermogravimetric analysis. The rare earth metal oxides obtained were characterized for the nature of chemical bonds and textural properties using FTIR and N2-physisorption analyses, respectively. The FTIR analysis of the rare earth metal precursors and the oxides showed that the OH– and NO– bonds depicting the presence of hydrated water molecules and nitrate disappeared after the thermal decomposition leaving out only the pure solid oxides. The kinetics data obtained from the thermogravimetric analysis were fitted into “model free” kinetic expressions such as Kissinger, Ozawa–Flynn–Wall to calculate the apparent activation energy of the solid-state decomposition reaction of the rare earth metal precursors. The kinetic parameters were further analyzed using Coat–Redfern model to determine the possible mechanism of the decomposition process. The calculated values of the activation energy obtained from both Kissinger and Ozawa–Flynn–Wall models were similar compared to that obtained from Coat–Redfern model. Highest activation energies of 230.26, 344.78, 320.2.78, 392.72 and 258.26 kJ mol−1 were obtained from decomposition of Ce(NO3)3·6H2O, La(NO3)3·6H2O, Sm(NO3)3·6H2O, Nd(NO3)3·6H2O and Pr(NO3)3·6H2O), respectively, using Kissinger model, while the analysis of the kinetic data using Ozawa–Flynn–Wall model gave the highest activation energies of 229.01, 350.56, 348.56, 392.72 and 388.56 kJ mol−1 for decomposition of Ce(NO3)3·6H2O, La(NO3)3·6H2O, Sm(NO3)3·6H2O, Nd(NO3)3·6H2O and Pr(NO3)3·6H2O), respectively. Thirteen different models were evaluated using Coat–Redfern models in order to determine the mechanisms that govern the decomposition process. Interestingly, two-dimensional diffusion mechanism with activation energy of 105.61, 107.61, 140.61, 144.52 and 154.78 kJ mol−1 was obtained for thermal decomposition of Ce(NO3)3·6H2O, La(NO3)3·6H2O, Sm(NO3)3·6H2O, Nd(NO3)3·6H2O and Pr(NO3)3·6H2O), respectively. The rare earth metal oxides obtained from this study finds potential application as supports, promoters and catalysts in the field of catalysis.



Similar content being viewed by others
References
Hedrick JB. Rare-earth metals. US Geol Surv 1998:61.1–61.12. http://minerals.usgs.gov/minerals/pubs/commodity/rare_earths/740497.pdf.
Alonso E, Sherman AM, Wallington TJ, Everson MP, Field FR, Roth R, et al. Evaluating rare earth element availability: a case with revolutionary demand from clean technologies. Environ Sci Technol. 2010;46:3406–14. doi:10.1021/es203518d.
Sato S, Takahashi R, Kobune M, Gotoh H. Basic properties of rare earth oxides. Appl Catal A Gen. 2009;356:57–63. doi:10.1016/j.apcata.2008.12.019.
Igarashi A, Ichikawa N, Sato S, Takahashi R, Sodesawa T. Dehydration of butanediols over CeO2 catalysts with different particle sizes. Appl Catal A Gen. 2006;300:50–7. doi:10.1016/j.apcata.2005.10.054.
Sato S, Takahashi R, Kobune M, Inoue H, Izawa Y, Ohno H, et al. Dehydration of 1,4-butanediol over rare earth oxides. Appl Catal A Gen. 2009;356:64–71. doi:10.1016/j.apcata.2008.12.017.
Igarashi A, Sato S, Takahashi R, Sodesawa T, Kobune M. Dehydration of 1,4-butanediol over lanthanide oxides. Catal Commun. 2007;8:807–10. doi:10.1016/j.catcom.2006.09.003.
Sato S. Dehydration of 1,4-butanediol into 3-buten-1-ol catalyzed by ceria. Catal Commun. 2004;5:397–400. doi:10.1016/j.catcom.2004.05.006.
Ayodele BV, Khan MR, Cheng CK. Syngas production from CO2 reforming of methane over ceria supported cobalt catalyst: effects of reactants partial pressure. J Nat Gas Sci Eng. 2015;. doi:10.1016/j.jngse.2015.09.049.
Therdthianwong S, Therdthianwong A, Siangchin C, Yongprapat S. Synthesis gas production from dry reforming of methane over Ni/Al2O3 stabilized by ZrO2. Int J Hydrogen Energy. 2008;33:991–9. doi:10.1016/j.ijhydene.2007.11.029.
Laosiripojana N, Sutthisripok W, Assabumrungrat S. Synthesis gas production from dry reforming of methane over CeO2 doped Ni/Al2O3: influence of the doping ceria on the resistance toward carbon formation. Chem Eng J. 2005;112:13–22. doi:10.1016/j.cej.2005.06.003.
Zhang B, Cai W, Li Y, Xu Y, Shen W. Hydrogen production by steam reforming of ethanol over an Ir/CeO2 catalyst: reaction mechanism and stability of the catalyst. Int J Hydrogen Energy. 2008;33:4377–86. doi:10.1016/j.ijhydene.2008.05.022.
Xu W, Si R, Senanayake SD, Llorca J, Idriss H, Stacchiola D, et al. In situ studies of CeO2-supported Pt, Ru, and Pt–Ru alloy catalysts for the water–gas shift reaction: active phases and reaction intermediates. J Catal. 2012;291:117–26. doi:10.1016/j.jcat.2012.04.013.
Wang H, Liu Y, Wang L, Qin YN. Study on the carbon deposition in steam reforming of ethanol over Co/CeO2 catalyst. Chem Eng J. 2008;145:25–31. doi:10.1016/j.cej.2008.02.021.
Verykios XE. Catalytic dry reforming of natural gas for the production of chemicals and hydrogen. Int J Hydrogen Energy. 2003;28:1045–63. doi:10.1016/S0360-3199(02)00215-X.
Abasaeed AE, Al-fatesh AS, Naeem MA, Ibrahim AA, Fakeeha AH. Catalytic performance of CeO2 and ZrO2 supported Co catalysts for hydrogen production via dry reforming of methane. Int Hydrogen Energy. 2015:6818–26. doi:10.1016/j.ijhydene.2015.03.152.
Ayodele BV, Cheng CK. Modelling and optimization of syngas production from methane dry reforming over ceria-supported cobalt catalyst using artificial neural networks and Box–Behnken design. J Ind Eng Chem. 2015;. doi:10.1016/j.jiec.2015.08.021.
Ayodele BV, Khan MR, Lam SS, Cheng CK. Production of CO-rich hydrogen from methane dry reforming over lanthania-supported cobalt catalyst: kinetic and mechanistic studies. Int J Hydrogen Energy. 2016;. doi:10.1016/j.ijhydene.2016.01.091.
Ayodele BV, Khan MR, Cheng CK. Catalytic performance of ceria-supported cobalt catalyst for CO-rich hydrogen production from dry reforming of methane. Int J Hydrogen Energy. 2015;. doi:10.1016/j.ijhydene.2015.10.049.
Wendlandt W. Thermal decomposition of scandium, yttrium, and rare earth metal oxalates. Anal Chem. 1958;30:58–61.
Wendlandt WW. The thermal decomposition of the heavier rare earth metal chloride hydrates. J Inorg Nucl Chem. 1959;9:136–9. doi:10.1016/0022-1902(59)80072-5.
Wendlandt WW. The thermal decomposition of yttrium, scandium, and some rare-earth chloride hydrates. J Inorg Nucl Chem. 1957;5:118–22. doi:10.1016/0022-1902(57)80052-9.
Wendlandt WW. The thermolysis of the rare earth and other metal nitrates. Anal Chim Acta. 1956;15:435–9. doi:10.1016/0003-2670(56)80082-2.
Im G. Low-temperature molar heat capacity and thermodynamic properties of rare earth complex. J Therm Anal Calorim. 2016;124:429–35. doi:10.1007/s10973-015-5133-8.
Zapa BL, Zapa W. Synthesis, spectral and thermal study of La (III), Nd(III), Sm(III), Eu(III), Gd(III) and Tb(III) complexes with mefenamic acid. J Therm Anal Calorim. 2016:363–74. doi:10.1007/s10973-015-5120-0.
Novikov VV, Mitroshenkov NV, Matovnikov AV, Avdashchenko DV, Trubnickov SV, Morozov AV. Peculiarities of the lattice thermal properties of rare-earth tetraborides. J Therm Anal Calorim. 2015:1597–602. doi:10.1007/s10973-015-4475-6.
Hussein GAM. Rare earth metal oxides: formation, characterization and catalytic activity thermoanalytical and applied pyrolysis review. J Anal Appl Pyrolysis. 1996;37:111–49. doi:10.1016/0165-2370(96)00941-2.
Blaine RL, Kissinger HE. Homer Kissinger and the Kissinger equation. Thermochim Acta. 2012;540:1–6. doi:10.1016/j.tca.2012.04.008.
Aboulkas A, El harfi K, El Bouadili A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: pyrolysis kinetics and mechanisms. Energy Convers Manag. 2010;51:1363–9. doi:10.1016/j.enconman.2009.12.017.
Ebrahimi-Kahrizsangi R, Abbasi MH. Evaluation of reliability of Coats–Redfern method for kinetic analysis of non-isothermal TGA. Trans Nonferrous Metals Soc China. 2007;18:2–6.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6. doi:10.1246/bcsj.38.1881.
Yao F, Wu Q, Lei Y, Guo W, Xu Y. Thermal decomposition kinetics of natural fibers: activation energy with dynamic thermogravimetric analysis. Polym Degrad Stab. 2008;93:90–8. doi:10.1016/j.polymdegradstab.2007.10.012.
Melnikov P, Arkhangelsky IV, Nascimento VA, Silva AF, Consolo LZZ. Thermolysis mechanism of samarium nitrate hexahydrate. J Therm Anal Calorim. 2014:1537–41. doi:10.1007/s10973-014-4067-x.
Strydom C, van Vuuren CP. The thermal decomposition of Cerium(I11) nitrate hydrate. J Therm Anal. 1987;32:157–60.
Hussein GAM. Formation of praseodymium oxide from the thermal decomposition of hydrated praseodymium acetate and oxalate. J Anal Appl Pyrolysis. 1994;29:89–102. doi:10.1016/0165-2370(93)00782-I.
Balboul BAA, Myhoub AYZ. The characterization of the formation course of neodymium oxide from different precursors: a study of thermal decomposition and combustion processes. J Anal Appl Pyrolysis. 2010;89:95–101. doi:10.1016/j.jaap.2010.06.003.
Ihli J, Wong WC, Noel EH, Kim Y-Y, Kulak AN, Christenson HK, et al. Dehydration and crystallization of amorphous calcium carbonate in solution and in air. Nat Commun. 2014;5:3169. doi:10.1038/ncomms4169.
Arii T, KIshi A, Ogawa M, Sawada Y. Thermal decomposition of cerium (III) acetate hydrate by a three-dimensional thermal analysis. Anal Sci. 2001;17:875.
Premkumar T, Govindarajan S, Coles AE, Wight CA. Thermal decomposition kinetics of hydrazinium cerium 2,3-pyrazinedicarboxylate hydrate: a new precursor for CeO2. J Phys Chem B. 2005;109:6126–9. doi:10.1021/jp0445223.
Todorovsky DS, Getsova MM, Vasileva MA. Thermal decomposition of lanthanum-titanium citric complexes prepared from ethylene glycol medium. J Mater Sci. 2002;37:4029–39. doi:10.1023/A:1019600815906.
Hussein GAM, Buttrey DJ, DeSanto P, Abd-Elgaber AA, Roshdy H, Myhoub AYZ. Formation and characterization of samarium oxide generated from different precursors. Thermochim Acta. 2003;402:27–36. doi:10.1016/S0040-6031(02)00535-X.
Kȩpiński L, Zawadzki M, Miśta W. Hydrothermal synthesis of precursors of neodymium oxide nanoparticles. Solid State Sci. 2004;6:1327–36. doi:10.1016/j.solidstatesciences.2004.07.003.
Acknowledgements
The authors would like to acknowledge the research fund RDU130501 granted by the Ministry of Science, Technology and Innovation Malaysia (MOSTI) and the DSS scholarship granted to Bamidele V. Ayodele by the Universiti Malaysia Pahang.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ayodele, B.V., Hossain, M.A., Chong, S.L. et al. Non-isothermal kinetics and mechanistic study of thermal decomposition of light rare earth metal nitrate hydrates using thermogravimetric analysis. J Therm Anal Calorim 125, 423–435 (2016). https://doi.org/10.1007/s10973-016-5450-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5450-6