Abstract
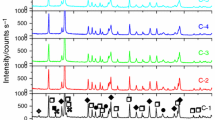
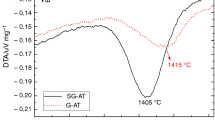
The non-isothermal kinetics of cordierite formation from both non-activated and mechanically activated kaolinite + talc + alumina ceramic system was studied by differential thermal analysis (DTA). The mixture of kaolinite, talc and alumina was activated mechanically in a planetary mill, while amorphisation in the kaolinite, talc and alumina structure was studied by X-ray diffraction analysis. The activation energies depending on the conversion for cordierite formation were calculated from the DTA curves by using the non-isothermal method of Coats and Redfern at heating rates of 5, 10, 15 and 20 °C min−1. The mechanical activation of the kaolinite, talc and alumina mixture resulted in the decrease in activation energy values for cordierite formation.







Similar content being viewed by others
References
Obradović N, Đorđević N, Filipović S, Nikolić N, Kosanović D, Mitrić M, Marković S, Pavlović V. Influence of mechanochemical activation on the sintering of cordierite ceramics in the presence of Bi2O3 as a functional additive. Powder Technol. 2012;218:157–61.
Orosco P, Ruiz M del C, Gonzalez J. Synthesis of cordierite by dolomite and kaolinitic clay chlorination. Study of the phase transformations and reaction mechanism. Powder Technol. 2014;267:111–8.
Gökçe H, Ağaoğulları D, Öveçoğlu ML, Duman İ, Boyraz T. Characterization of microstructural and thermal properties of steatite/cordierite ceramics prepared by using natural raw materials. J Eur Ceram Soc. 2011;31:2741–7.
Kobayashi Y, Sumi K, Kato E. Preparation of dense cordierite ceramics from magnesium compounds and kaolinite without additives. Ceram Int. 2000;26:739–43.
Yalamaç E, Akkurt S. Additive and intensive grinding effects on the synthesis of cordierite. Ceram Int. 2006;32:825–32.
Ghitulica C, Andronescu E, Nicola O, Dicea A, Biran M. Preparation and characterization of cordierite powders. J Eur Ceram Soc. 2007;27:711–3.
Tunç T, Demirkıran AŞ. The effects of mechanical activation on the sintering and microstructural properties of cordierite produced from natural zeolite. Powder Technol. 2014;260:7–14.
Tamborenea S, Mazzoni AD, Aglietti EF. Mechanochemical activation of minerals on the cordierite synthesis. Thermochim Acta. 2004;411:219–24.
Vyazovkin S. Isoconversional kinetics. In: Brown ME, Gallagher PK, editors. Handbook of thermal analysis and calorimetry. Amsterdam: Elsevier BV; 2008. p. 508–38.
Balaz P. Mechanochemistry in nanoscience and minerals engineering. Berlin: Springer; 2008.
Tromans D, Meech JA. Enhanced dissolution of minerals: microtopography and mechanical activation. Miner Eng. 1999;12(6):609–25.
Tromans D, Meech JA. Enhanced dissolution of minerals: stored energy, amorphism and mechanical activation. Miner Eng. 2001;14(11):1359–77.
Koç S, Toplan N, Yıldız K, Topaln HÖ. Effects of mechanical activation on the non-iso-thermal kinetics of mullite formation from kaolinite. J Therm Anal Calorim. 2011;103:791–6.
Elmas E, Toplan N, Yıldız K, Toplan HÖ. The non-isothermal kinetics of mullite formation in mechanically activated kaolinite-alumina ceramic system. J Therm Anal Calorim. 2012;108:1201–6.
Frost RL, Horvath E, Mako E, Kristof J, Redey A. Slow transformation of mechanically dehydroxylated kaolinite to kaolinite—an aged mechanochemically activated formadide-intercalated kaolinite study. Thermochim Acta. 2003;408:103–13.
Miller JG, Oulton TD. Prototropy in kaolinite during percussive grinding. Clay Clay Miner. 1970;18(6):313–23.
Benito JM, Turrillas X, Cuello GJ, De Aza AH, De Aza S, Rodrigez MA. Cordierite synthesis, a time-resolved neutron diffraction study. J Eur Ceram Soc. 2012;32:371–9.
Naskar MN, Chatterjee M. A novel process for the synthesis of cordierite (Mg2Al4Si5O18) powders from rice husk ash and other sources of silica and their comparative study. J Eur Ceram Soc. 2004;24:3499–508.
Tkacova K. Mechanical activation of minerals. Amsterdam: Elsevier; 1989.
Boldyrev VV, Tkacova T. Mechanochemistry of solids: past, present, and prospects. J Mater Synth Proc. 2000;8(3–4):121–32.
Steinike U, Tkacova K. Mechanochemistry of solids—real structure and reactivity. J Mater Synth Proc. 2000;8(3–4):197–203.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yürüyen, S., Toplan, N., Yildiz, K. et al. The non-isothermal kinetics of cordierite formation in mechanically activated talc–kaolinite–alumina ceramics system. J Therm Anal Calorim 125, 803–808 (2016). https://doi.org/10.1007/s10973-016-5277-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5277-1