Abstract
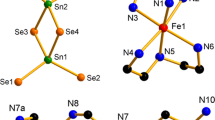
Using hydrothermal synthesis, Hf(SO4)2(H2O)4 and Hf(SeO3)(SeO4)(H2O)4 were obtained and characterized by X-ray, IR spectroscopy and thermo-gravimetric analysis. The experimental data from the IR spectra of the compounds showed characteristic absorption bands for the sulfate, selenite and selenate anions. The dehydration of the two complexes proceeded in two stages in which three and one water molecules were released, respectively. The decomposition up to 850 °C did not give HfO2, but lead to formation of intermediate phases Hf2O3(SO4) and Hf2O3(SeO3). The decomposition of the mixed valence selenite-selenate proceeds by two mechanisms, the first one resulting in the formation of intermediate HfO(SeO3) and the second one—its decomposition and formation of dihafnyl selenite Hf2O3(SeO3). The activation energy and the pre-exponential factor in the Arrhenius equations were determined for each process of dehydration and decomposition. These values were quite high for the decomposition of HfO(SeO3) due to the very strong bonds between the HfO2+ and SeO3 2− ions.




Similar content being viewed by others
References
Bear IJ, Mumme WG. The preparation and characterization of phases in the Hf(SO4)2–H2O system. J Inorg Nucl Chem. 1970;32:1159–64.
Ahmed MAK, Fjellvag H, Kjekshus A. Synthesis and characterization of zirconium and hafnium sulfates, hydroxide sulfates and oxide sulfates. Acta Chem Scand. 1999;53:24–33.
Nabar MA, Ajgaonkar VR. Studies on selenates. III. Crystal chemical data for zirconium and cerium selenate tetrahydrates. J Appl Crystallogr. 1978;11:56–7.
Yankova R, Genieva S, Halachev N, Dimitrova G. Synthesis and quantumchemical study on Hf(SO4)2(H2O)4 and Hf(SeO4)2(H2O)4 complexes. Am J Chem. 2014;4:125–9.
Lee EP, Song SY, Lee DW, Ok KM. New bismuth selenium oxides: syntheses, structures, and characterizations of centrosymmetric Bi2(SeO3)2(SeO4) and Bi2(TeO3)2(SeO4) and noncentrosymmetric Bi(SeO3)(HSeO3). Inorg Chem. 2013;52:4097–103.
Wickleder MS, Buchner O, Wickleder C, el Sheik S, Brunklaus G, Eckert H. Au2(SeO3)2(SeO4): synthesis and characterization of a new noncentrosymmetric selenate-selenate. Inorg Chem. 2004;43:5860–4.
Weil M, Kolitsch U. Hg3Se3O10, a mercury(II) compounds with mixed-valence oxoselenium (IV/VI) anions. Acta Crystallogr C. 2002;58:i47–9.
Song SY, Lee DW, Ok KM. Rich structural chemistry in scandium selenium/tellurium oxides: mixed-valent selenite-selenates, Sc2(SeO3)2(SeO4) and Sc2(TeO3)(SeO3)(SeO4), and ternary tellurite, Sc2(TeO3)3. Inorg Chem. 2014;53:7040–6.
Feng ML, Mao JG. Synthesis and crystal structure of a new neodymium(III) selenate-selenite: Nd2(SeO4)(SeO3)2(H2O)2. J Alloys Compd. 2005;388:23–7.
Berdonosov PS, Schmidt P, Dityat’yev OA, Dolgikh VA, Lightfoot P, Ruck M. Nd2(SeO3)2(SeO4)·2H2O—a mixed-valence compound containing selenium in the oxidation states +IV and +VI. Z Anorg Allg Chem. 2004;630:1395–400.
Morris RE, Wilkinson AP, Cheetam AK. A novel mixed-valence selenium(IV)/Selenium(VI) oxo compound: crystal structure determination and X-ray absorption near edge structure study of Erbium selenate(IV) Selenate(VI) hydrate, Er(SeO3) (SeO4)1/2·H2O. Inorg Chem. 1992;31:4774–7.
Jin GB, Skanthakumar S, Soderholm L. Two new neptunyl(V) selenites: a novel cation-cation interaction framework in (NpO2)3(OH)(SeO3)(H2O)2·H2O and a Uranophane-type sheet in Na(NpO2)(SeO3)(H2O). Inorg Chem. 2011;50:6297–303.
Yankova R, Genieva S, Halachev N, Dimitrova G. Molecular structure, vibrational spectra, MEP, HOMO-LUMO and NBO analysis of Hf(SeO3)(SeO4)(H2O)4. J Mol Struct. 2016;1106:82–8.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery Jr JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennu-cci B, Cossi M, Scalmani G, Rega N, Peter-sson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Strat-mann RE, Yazyev O, Austin AJ, Cammi R, Po-me-lli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Za-krzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Rag-havachari K, Foresman JB, Ortiz JV, Cui Q, Ba-boul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, -Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gon-za-lez C, Pople JA. Gaussian 03, Revision B.04, Gaussian, Inc., Wallingford CT, 2004.
Yankova R, Halachev N, Baikusheva-Dimitrova G, Genieva S. Synthesis and quantumchemical study on hafnium compounds. Sci Technol. 2014;4:64–9.
Sohn JR, Seo DH, Lee SH. Acid properties and catalytic activities of titanium sulfate supported on γ-alumina. J Ind Eng Chem. 2004;10:309–15.
Sohn JR, Kim YT, Shin DC. NiSO4 supported on FeO-promoted ZrO2 catalyst for ethylene dimerization. Bull Korean Chem Soc. 2005;26:1749–56.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetric data. Nature (London). 1964;201:68–9.
Vlaev LT, Georgieva VG, Genieva SD. Kinetic parameters of decomposition of some selenates. Generalized perturbation theory of chemical reactivity. J Therm Anal Cal. 2006;83:421–7.
Vlaev L, Nedelchev N, Gyurova K, Zagorcheva M. A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. J Anal Appl Pyrolysis. 2008;81:253–62.
Sestak J. Thermophysical properties of solids. Academica: Prague; 1984.
Vlaev LT, Georgieva VG, Genieva SD. Products and kinetics of non-isothermal decomposition of vanadium(IV) oxide compounds. J Therm Anal Cal. 2007;88:805–12.
Wickleder MS. Oxo-selenates of rare earth elements. In: Gshneidner KA Jr, Bünzli Y-CG, Percharsky VK (eds) Handbook on the physic and chemistry of rare earths. Elsevier 2005. pp. 42–87.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Genieva, S., Yankova, R., Baikusheva-Dimitrova, G. et al. Synthesis and characterization of Hf(SO4)2(H2O)4 and Hf(SeO3)(SeO4)(H2O)4 . J Therm Anal Calorim 124, 1595–1600 (2016). https://doi.org/10.1007/s10973-016-5275-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5275-3