Abstract
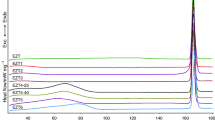
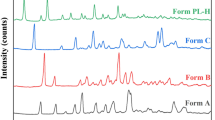
Polymorphism is the ability of a substance to crystallize into different crystalline states. Different polymorphs vary according to their physical and chemical properties, and therefore, the presence of different crystalline forms of a drug may change some physicochemical properties of certain medicines. The goal of this work was to detect polymorphic forms of a compound used as a drug, bromopride. For the purpose of this study, recrystallizations were carried out from different solvents and at distinct temperatures, with the aim of obtaining different crystalline forms, which were characterized by dynamic thermal analysis (DTA) and X-ray powder diffraction. The samples obtained through recrystallization in different temperature conditions and solvents showed peculiar DTA curves and diffractograms profiles, indicating the presence of crystalline forms distinct from each other.





Similar content being viewed by others
References
Giron D. Thermal analysis and calorimetric methods in the characterization of polymorphs and solvates. Thermochim Acta. 1995;248:1–59.
Bottom R. The role of modulated temperature differential scanning calorimetry in the characterization of a drug molecule exhibiting polymorphic and glass forming tendencies. Int J Pharm. 1999;192:47–53.
Galvão WG. Carbamazepina no estado sólido e sua susceptibilidade polimórfica. 2009. Master’s thesis. 2009.
Giron D, Goldbronn C. Use of DSC and TG for identification and qualification of the dosage form. J Therm Anal Calorim. 1997;49:907–12.
Toscani S. An up-to-date approach to drug polymorphism. Therm Acta. 1998;321:73–9.
Giron D, Goldbronn C, Mutz M, Pfeffer S, Piechon P, Schwab P. Solid-state characterizations of pharmaceutical hydrates. J Therm Anal Calorim. 2002;68:453–65.
Shekunov BY, York P. Crystallization process in pharmaceutical technology and drug delivery design. J Cryst Growth. 2000;211:122–36.
Therefall T. Crystallization of polymorphs: thermodynamic insight into the role of solvent. Org Process Res Dev. 2000;4:384–90.
Kitamura M. Controlling factor of polymorphism in crystallization process. J Cryst Growth. 2002;237:2205–14.
Yua LX, Lionbergera RA, Rawa AS, D’Costa R, Wub H, Hussain AS. Applications of process analytical technology to crystallization process. Adv Drug Deliv Rev. 2004;56:349–69.
Schroer JW, Ng KM. Process paths of kinetically controlled crystallization: enantiomers and polymorphs. Ind Eng Chem Res. 2003;42:2230–44.
Aulton ME. Delineamento de formas farmacêuticas. 2005.
Rustichelli C, Gamberini G, Ferioli V. Solid-state study of polymorphic drugs: carbamazepine. J Pharm Biomed Anal. 2000;23:41–54.
Mcgregor C, Saunder MH, Buckton G, Saklatvala R. The use of high speed differential scanning calorimetry (Hiper-DSC) to study the thermal properties of carbamazepine polymorphs. Thermochim Acta. 2004;417:231–7.
Rodrigues-spong B, Price CP, Jayasankar A, Matzger AJ, Rodrigues-Hornedo N. General principles of pharmaceutical solid polymorphism: a supramolecular perspective. Adv Drug Deliv Rev. 2004;56:241–74.
Porter WW III, Elie SC, Matzger AJ. Polymorphism in carbamazepine cocrystals. Cryst Growth Des. 2008;8:14–6.
Souza FS, Basilio ID Jr, Oliveira EJ, Macedo RO. Correlation studies between thermal and dissolution rate constants of cimetidine drug and tablets. J Therm Anal Calorim. 2003;72:549–54.
Souza MAF, Conceição MM, Silva MCD, Soledade LEB, Souza AG. Thermal and kinetic study of statins. J Therm Anal Calorim. 2007;87:859–63.
Farmacopeia Brasileira. 5ª edição: Volume I. Brasília, 2010.
Lima LS, Weinert PL, Pezza L, Pezza HR. Sensitive flow-injection spectrophotometric analysis of bromopride. Spectrochim Acta, Part A. 2014;133:597–604.
Stulzer HK, Rodrigues PO, Cardoso TM, Matos JSR, Silva MAS. Compatibility studies between captopril and pharmaceutical excipients used in tablets formulations. J Therm Anal Calorim. 2008;91:323–8.
Saraswat S, Sharma SD. Correlation between glass transition and crystallization phenomena in context of Meyer–Neldel rule. J Therm Anal Calorim. 2014;117:1263–70.
Paulino AS, Rauber GS, Campos CEM, Maurício MHP, Avillez RR, Cuffini SL, Cardoso SG. Hollow crystal anti-solvent preparation process as a promising technique to improve dissolution of poorly soluble drugs. J Cryst Growth. 2013;366:76–81.
Yang M, Gogos C. Crystallization of poly(ethylene oxide) with acetaminophen—a study on solubility, spherulitic growth, and morphology. Eur J Pharm Biopharm. 2013;85:889–97.
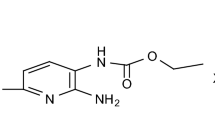
Tanaka R, Hirayama I. Structure of bromopride. Anal Sci: X-Ray Struct Anal Online. 2004;20:79–80.
Acknowledgements
The authors are grateful to Professor Dr. Costa of State University of Maringa for XRPD analysis. J. Cortez is grateful to CAPES for postdoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carrer, H., Cortez, J., Frare, L.M. et al. Thermal characterization of the bromopride recrystallized from different solvents and at different temperature conditions. J Therm Anal Calorim 123, 927–931 (2016). https://doi.org/10.1007/s10973-015-5027-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-5027-9