Abstract
As a healthcare-associated pathogen with the resistance to anti-bacterial agent, P. aeruginosa has caused prevalent public burden and should not be ignored. Facing the realistic situation, developing novel anti-P. aeruginosa agents is urgent. Leaves of Dracontomelon dao were extensively used in southern China to treat various infectious diseases 1000 years ago. And in the study, the heat flow power–time (HFP–time) curves generated by P. aeruginosa which were disturbed by the five fractions (PE, CHCl3, EtOAc, n-BuOH and Vestiges fraction) from them were investigated through microcalorimetry, and then, some thermal kinetic parameters were obtained from the curves to characterize the metabolism of P. aeruginosa. The parameters were analyzed by principal component analysis (PCA), and the anti-P. aeruginosa activities of the five fractions were systematically compared and evaluated. The results showed that five fractions all expressed respective anti-P. aeruginosa effects in a dose-dependent manner and especially the performance of EtOAc fraction with half-inhibitory concentration (IC50) of 18.06 μg mL−1. Simultaneously, the prospective performance of EtOAc fraction was confirmed by turbidimetry. Based on the promising anti-P. aeruginosa activities of EtOAc fraction, it could be developed as a novel anti-bacterial agent used in practice for treating certain infectious diseases.
Similar content being viewed by others
Introduction
Pseudomonas aeruginosa, well-known for possessing a characteristically large genome with metabolic versatility [1], is a classic model organism forming the biofilm [2]. However, it is also the second most frequent pathogen causing ventilator-associated pneumonia and the third or fourth most frequent pathogen causing septicemia, urinary tract infections and surgical wound infections among gram-negative pathogens [3]. The major presentations of P. aeruginosa infection are that bacteremia in the immunocompromised, pneumonia in cystic fibrosis patients, community-acquired ear and pneumonia infections, and hospital-acquired outbreaks principally associated with contaminated solutions or medical devices among general patients or those in intensive care units (ICUs) [4, 5]. And the nosocomial pneumonia deaths caused by P. aeruginosa were estimated to be approximately 1400 per year in the USA according to a meta-analysis of 43 outbreaks in hospitals covering 35 years (1966–2001) [6]. In addition, P. aeruginosa was responsible for 15.6 % of all nosocomial pneumonia cases in medical–surgical ICUs [7]. A set of epidemiological data about patients with P. aeruginosa in the ICUs over a 10-year period illustrated that approximately 4.0 % of ICU admissions were colonized or infected by P. aeruginosa (1998–2007) [8]. It was also found to be the most frequent gram-negative bacterium recovered from patients with nosocomial pneumonia during ICU surveillance over the last two decades [9]. It is even worse that the overall rate of nosocomial P. aeruginosa infections has increased rapidly in some patient groups [10]. Moreover, P. aeruginosa was the leading pathogen (17 %) responsible for all types of infections including sepsis, urinary tract infection and pneumonia (2006–2010) [11]. P. aeruginosa has caused serious burden to public health care and safety with the characteristic of drug resistance in recent years [12–15]. Facing the actual situation, discovering novel anti-P. aeruginosa agents is urgent and realistic.
Currently, the plant extracts or their constituents of prospective anti-bacterial activities were drawn more and more attraction [16]. As a medicinal material, the leaves of Dracontomelon dao classified into anacardiaceae were widely used for treating various infectious diseases, such as decubitus and skin ulcer. Anti-bacterial effects of the leaves of Dracontomelon dao are objective and authentic based on its clinical practice in southern China since Jin dynasty. However, the available foundational research report about the Dracontomelon dao is extremely limited. Existing studies have shown that the crude methanol extracts of the leaves, stem and root barks of Dracontomelon dao and their subsequent fractions demonstrated a broad anti-bacterial spectrum [17]. Subsequently, some researches have indicated that the extract fraction from the leaves of Dracontomelon dao exerts a potential anti-Escherichia coli and anti-Staphylococcus aureus activity, especially the EtOAc fraction [18, 19]. Comprehensively, the leaves of Dracontomelon dao may possess the promising ability of anti-P. aeruginosa and it can be implied that the different fractions from the leaves of Dracontomelon dao could become a novel anti-P. aeruginosa agent. It is essential to effectively evaluate the anti-P. aeruginosa activities of different extracted fractions.
Microcalorimetry used in monitoring bio-system metabolic processes is an objective and sensitive method in evaluating biological activity. It has many distinctive advantages, such as directly detecting the bio-system thermal effects to obtain the thermodynamic information like thermodynamic multi-parameter and curve in a non-disturbing and noninvasive way. Moreover, the metabolic state could be monitored in a continuous, online and successive style to determine the strength of drug or chemicals. Finally, the microcalorimetry has the characteristic of high throughput with simple pretreatment to bio-system, including cell, bacterium and organelles [20–22]. The admirable method is widely used in medical classification and environmental monitoring [23, 24], especially in evaluating bioactive fractions or components of medicinal material in recent years [18, 19, 25].
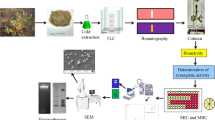
To solve the problem fast and efficiently, we specifically investigated and evaluated the anti-P. aeruginosa activities of five fractions from the leaves of Dracontomelon dao. The whole experimental process is demonstrated in Fig. 1. We found that in five fractions, four of them exhibited potential effects against P. aeruginosa with EtOAC fraction being the strongest, except n-BuOH fraction. The IC50 of EtOAC fraction was 18.06 μg mL−1, the lowest among the four effective fractions. Furthermore, the MIC of EtOAC fraction was figured out to be 10 μg mL−1. These discoveries indicated that the EtOAC fraction of the leaves of Dracontomelon dao could exert a potent anti-P. aeruginosa effect and was potential to be a novel therapeutical alternative against P. aeruginosa or other infectious pathogens. It could be applied in treating certain bacterial infections in vitro.
Materials and methods
Bacterial strain and recovery
Pseudomonas aeruginosa (ATCC 27853) was provided by Clinical Examination Center of 302 Hospital of People’s Liberation Army (PLA), Beijing, China, and was inoculated into 25 mL Luria–Bertani (LB) culture medium, and it was incubated in 37 °C incubator shaker for 4 h at the rotational speed of 110 rpm and then was stored in a refrigerator at 4 °C.
Culture medium
The LB culture medium containing 10.0 g peptone, 5.0 g yeast extract and 5.0 g NaCl was dissolved in 1000 mL deionized water (pH 7.0–7.2), and the Mueller-Hinton B (M-H (B)) culture medium containing 17.5 g casein hydrolysates, 1.5 g starch and 5.0 g beef extract powder was dissolved in 1000 mL deionized water (pH 7.0–7.2). Later, the culture medium was both sterilized by autoclave at 0.1 MPa and 121 °C for 30 min and conserved in a refrigerator at 4 °C.
Instruments and reagents
The real-time metabolic HFP–time curves of P. aeruginosa were monitored by the type of 3114/3236 thermal activity monitor (TAM) air isothermal microcalorimeter (Thermometric AB, Sweden) and were recorded by computer with dedicated software package [26]. The baseline stability of this instrument was 0.2 mW for 24 h. The type of Synergy H1 (BioTek, America) was introduced to establish the growth curve of the P. aeruginosa. It could perfect the detecting performance based on filters and dichroic color mirror optical system, and it is more fast and sensitive than the grating system. Ultra-pure water in experiments was obtained from a Milli-Q Plus system (Bedford, MA, USA). The solvents (AR) used in the extraction experiment including the petroleum ether (PE; batch no. 2014101030), chloroform (CHCl3; 20140312), ethyl acetate (EtOAc; 20141016) and n-butyl alcohol (n-BuOH; 20140121) were made by Beijing Chemical Factory (Beijing, China).
Samples preparation
The leaves of Dracontomelon dao (batch NO. 20141013) purchased in Guangdong Province in China were identified by Professor Xiaohe Xiao (PLA Institute of Chinese Material, 302 Hospital of PLA, Beijing, 100039, P. R. China). 1000.0 g powder of the leaves of Dracontomelon dao was accurately weighed and refluxed 2 h with 12-fold 80 % ethanol. The procedure was repeated three times. Then, the first extract was filtered and evaporated. Furthermore, it was dissolved in ultra-pure water at the concentration of 0.1 g mL−1. Finally, it was further extracted by consequent solvent duplicated five times to get petroleum ether (PE), chloroform (CHCl3), ethyl acetate (EtOAc) and n-BuOH fraction at the proportion of 1:1.5. The yield of extracts was 5.93, 1.45, 4.18, 4.31 and 9.48 %, respectively. The five kinds of extraction liquids were low temperature dried and stored at 4 °C for the anti-P. aeruginosa experiments.
Microcalorimetric measurement
The microcalorimeter was thermostated at 37 °C using ampoule method. The five fractions from the leaves of Dracontomelon dao contained in 10.0 mL LB culture medium were added into 20.0 mL sterilized ampoule at different concentrations. Furthermore, each ampoule, except for the medium control group, should be guaranteed to contain the suspensions of P. aeruginosa at the cell density of 1.0 × 106 colony-forming units (CFU mL−1) in each milliliter. There were 30 min for pre-incubation before the thermodynamic curves were recorded. All signals reflecting thermodynamic information were collected by dedicated software package in a real-time manner. The whole process was operated on sterilized clean bench, and the equipment was cleaned by strict purge and high-temperature sterilization.
The multivariate statistical analysis of thermo-kinetic parameters
In most practical situations, variables are in a complicate correlation so it is demanded to capture the representative information. PCA aiming to reduce the dimension to narrow redundant and noise information from large and complex data sets and obtain important information responding to original data geography can ideally solve the problem. It is always used to simplify complex data sets and reduce the multi-dimension of data sets to determine the key variables, which may contribute to explain the differences in the observations and facilitate for further diagnosis and characterization. As a multivariate analysis method, PCA is commonly utilized in laboratory research, even clinical tests and epidemiological study [27–29]. PCA was employed to extract a set of orthogonal principal components (PCs) which accounted for the maximum variance in the data set in this study. Considering the units of original data were not uniform, correlation matrix possessing standardized function was used in PCA. Based on PCA, the unsupervised factor analysis focusing on interdependence of original variable within the correlation matrix contributed to descript the relationship between the original variables for explaining the anti-P. aeruginosa differences of the five fractions. Moreover, the supervised partial least squares discriminant analysis (PLS-DA) was coupled to understand more about the anti-P. aeruginosa activities of different fractions from the leaves of Dracontomelon dao. These useful statistical methods contributed to locate and develop novel anti-P. aeruginosa agent with high efficacy. All data were processed by SPSS statistics software version 20.0 and SIMCA-P 11.0.
The anti-P. aeruginosa of the EtOAc fraction using turbidimetry
The turbidimetry was used in this study to verify the interference of the novel fraction from leaves of Dracontomelon dao on the P. aeruginosa. The M-H (B) culture medium contained the suspensions of P. aeruginosa at the cell density of 1.0 × 105 CFU mL−1, and EtOAc fraction at different concentrations was added. The incubation circumstance included the temperature at 37 °C and the rotation speed of incubator shaker at 110 rpm. Samples and EtOAc fractions were added into five duplicates, while another three wells were set as accompanying blank control group by only adding M-H (B) culture medium and EtOAc fraction at corresponding concentrations to subtract the background interference. Then the optical density (OD) was obtained at 420 nm every 1 h [30]. All operation was repeated three times. It is worth noting that the OD values should be lower than 0.4, if not, the experimental group and accompanying blank control group should be diluted in reason.
Results
The normal HFP–time curve of P. aeruginosa
The normal HFP–time curve of P. aeruginosa without any exogenous intervention characterizing the growth of P. aeruginosa was formed by taking advantage of the ampoule method (Fig. 2). The curve always was divided into five phases [31]. From the HFP–time curve, the heat output signals were instructed by seven quantitative thermo-kinetic parameters, namely, k 1, k 2, P 1, P 2, t 1, t 2 and Q, in which k value represented growth rate constant and P value represented the two maximum heat output powers in the first and second exponential growth phase. In addition, t value was consistent with the peak time and Q value illustrating the two successive cup shape area was the total heat output. Finally, since ln P t and t fitting to linearity, the growth rate constant k representing exponential phase of metabolism of P. aeruginosa could be obtained from following Eq. 1 [32].
Five phases (I → V) reflecting the normal HFP–time curve of P. aeruginosa was obtained by microcalorimetric method. The five phases, respectively, were the lag phase (A → B), the first exponential growth phase (B → C), the stationary phase (C → D), the second exponential growth phase (D → E) and the decline phase (E → F)
The different subscripts of P are the heat output power at different corresponding time points.
The HFP–time curves of P. aeruginosa influenced by a certain concentration range of different fractions from the leaves of Dracontomelon dao
The metabolic characters of P. aeruginosa were disturbed when five different polar solvent extractions from leaves of Dracontomelon dao were added. Compared with the blank control groups, the height, shape and the time of peak were altered evidently of all experimental groups. All the features and diversifications of the metabolism characters of P. aeruginosa could be separated apparently within or between groups (Fig. 3), which indicated the five fractions had the activity of suppression even killing to P. aeruginosa. There were specific performances of the metabolism characters of P. aeruginosa. For instance, the time of peak was postponed and the height of peak was lowered accompanying with concentration increased (Fig. 3a, b and e). EtOAc fraction had anti-P. aeruginosa effects in a lowest concentration range (Fig. 3c), which might be an optimal anti-P. aeruginosa agent. Among all extract fractions, n-BuOH fraction, interestingly, even exerted an almost reverse effect at 3.2 mg mL−1 that it accelerated the time and increased the height of peak compared with the effect at 1.6 mg mL−1. However, it might still suppress the metabolism of P. aeruginosa because the first peak disappeared implying that the n-BuOH fraction might completely inhibit the growth of P. aeruginosa in the first exponential phase (Fig. 3d).
The statistical analysis of thermo-kinetic parameters and IC50 of different fractions
The HFP–time curves in Fig. 3 demonstrated an overall performance about the effects of five fractions on P. aeruginosa. And more information should be provided and processed to assess their anti-bacterial capabilities. Based on PCA, the quantitative thermo-kinetic parameters including k 1, t 1, P 1, k 2 , t 2, P 2 and Q obtained from the HFP–time curves were interpreted (Table 1). The results showed that k 2, t 2 and P 2 were the main parameters and far from the main cluster of others, which could represent the original datum and play an important role in evaluating and comparing the anti-P. aeruginosa activities (Fig. 4a). Considering the k 2 stands for the second exponential growth phase and is more objective with higher sensitivity, it was used for calculating inhibition rate I. So the growth inhibition ratio I (%) could be calculated on the basis of k 2 [27] and be defined as Eq. 2.
a Loadings plot indicated the contribution of the original variables (parameters) on the strength of the first two principal components PC1 and PC2. b Scores plot counted from unsupervised factor analysis showed the distribution of five fractions. In the plot, the first two scores T1 and T2 calculated from principal components and describing the variations under the premise of preserving the original information served as explaining the anti-P. aeruginosa activity of the five fractions. The clustering blue triangle in an ellipse was separated obviously from the others which represent the EtOAc fraction had hopeful anti-P. aeruginosa activity at different concentrations (0–30 μg mL−1). c Scores plot counted from the supervised PLS-DA in which blue diamond clustering in an ellipse also provided useful information to evaluate the novel anti-P. aeruginosa agents. d The IC50 of the five fractions. (Color figure online)
The k 0 represents the growth rate constant without any fraction, while the k c represents exponential constant with the fraction at different concentrations.
Furthermore, the IC50 could be, respectively, obtained. In addition, the original multiple data sets were analyzed by unsupervised factor analysis and supervised PLS-DA (Fig. 4b, c). The score plots indicated that five fractions had the stratification and cluster effect mutually, while the EtOAc fraction was separated obviously from the others. Furthermore, IC50 of EtOAc fraction was 18.06 μg mL−1 and the anti-bacterial effect of EtOAc fraction was the most potent from the remaining fractions (Fig. 5c, d). This phenomenon indicated that EtOAc fraction exhibited a more effective anti-P. aeruginosa activity at a lower concentration among five fractions.
The growth curves of P. aeruginosa presented at different concentrations of the EtOAc fraction
From the results of statistical analysis and IC50, EtOAc fraction could show good anti-P. aeruginosa effect in the range of low doses. To verify the good anti-bacterial activity and figure out the MIC of the EtOAc fraction, the turbidimetry was applied to establish the growth curves of P. aeruginosa influenced by the EtOAc fraction at different concentrations. On account of the exponential growth fitting with Eq. 1, the growth rate constant k could be quantized (Fig. 5a). When the concentration of the EtOAc fraction was increased, the growth rate constant was decreased in linear relationship (Fig. 5c), demonstrating that the anti-P. aeruginosa effect strengthened in a dose-dependent manner (Fig. 5b, c) with MIC being 10 μg mL−1. Combined with the result of IC50, it is favorable evidence that EtOAc fraction could settle the trouble to P. aeruginosa infectious diseases.
Discussion and conclusions
As an opportunistic pathogen, epidemiological studies have shown that P. aeruginosa leads to severe burden in public health care. At the same time, it causes various diseases, such as sepsis, urinary tract infection and pneumonia [8, 9]. In this study, we designed and investigated the anti-P. aeruginosa based on the activities of five fractions from leaves of Dracontomelon dao on the metabolism of P. aeruginosa using microcalorimetry combined with turbidimetry to rapidly and effectively screen the novel anti-P. aeruginosa agent.
Compared with the control groups, the P 1, P 2 and k 2 were decreased, the t 1 and t 2 were prolonged, and the inhibition rate I was increased generally when the concentrations of five fractions were increased, respectively. The total heat Q maintained the unchanged state because the available oxygen consumed by P. aeruginosa in each ampoule bottle was fix [2] (Table 1), which suggests that the five fractions even the medicinal material itself had the anti-P. aeruginosa activity and particularly the EtOAc fraction was prominent among five with the IC50 of 18.06 μg mL−1 (Table 1; Fig. 4b, d). Moreover, it could be considered that the sequence of anti-P. aeruginosa capability of the five was EtOAc fraction > CHCl3 fraction > PE fraction > vestiges fraction > n-BuOH fraction according to HFP–time curves and the IC50 (Figs. 3, 4d). Finally, the prospective performance of EtOAc fraction was confirmed by turbidimetry, and the MIC was about 10 μg mL−1. In summary, the EtOAc fraction had the strongest anti-bacterial activity in vitro [18, 19].
However, it is in a low incidence that patients are only infected by one type of bacteria. Mostly, patients are suffered from comprehensive interruption accompanying with multiple bacteria, drug-resistant bacteria, virus or fungi. Whether the leaves of Dracontomelon dao and its EtOAC fraction can exert an excellent anti-bacterial activity, confronting the infection from multi-bacteria, remains unknown, although it has a wide and strong anti-bacterial spectrum in vitro, which is also the shortage of the current research about anti-bacterial agents. And the situation is even more complicated in vivo, whether the EtOAc fraction can perform a strong anti-bacterial activity inside human body needs further evaluation. Based on the result of this current investigation, the conclusion could be obtained that EtOAc fraction from the leaves of Dracontomelon dao may be the novel anti-bacterial agent and used in practice for some infectious diseases, especially the traumatic infection.
It is worth mentioned that the microcalorimetry is a method that possesses non-destructive, noninvasive and quick advantages with high sensitivity from the total procedure of this case, which could be served as a new strategy for anti-bacterial drug discovery.
References
Masak J, Cejkova A, Schreiberova O, Rezanka T. Pseudomonas biofilms: possibilities of their control. FEMS Microbiol Ecol. 2014;89(1):1–14.
Zaharia DC, Muntean AA, Popa MG, Steriade AT, Balint O, Micut R, Iftene C, Tofolean I, Popa VT, Baicus C, Bogdan MA, Popa MI. Comparative analysis of Staphylococcus aureus and Escherichia coli microcalorimetric growth. BMC Microbiol. 2013;13:171–84.
Trautmann M, Bauer C, Schumann C, Hahn P, Höher P, Haller M, Lepper PM. Common RAPD pattern of Pseudomonas aeruginosa from patients and tap water in a medical intensive care unit. Int J Hyg Environ Health. 2009;209:325–31.
Falkinham JO, Hilborn ED, Arduino MJ, Pruden A, Edwards MA. Epidemiology and Ecology of Opportunistic Premise Plumbing Pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. Environ Health Perspect. 2015. PMID: 25793551.
Gattarello S, Ramírez S, Almarales JR, Borgatta B, Lagunes L, Encina B, Rello J. Causes of non-adherence to therapeutic guidelines in severe community-acquired pneumonia. Revista Brasileira de terapia intensiva. 2015;27(1):44–50.
Anaissie EJ, Penzak SR, Dignani MC. The hospital water supply as a source of nosocomial infections: a plea for action. Arch Intern Med. 2002;162:1483–92.
Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21(8):510–5.
Cuttelod M, Senn L, Terletskiy V, Nahimana I, Petignat C, Eggimann P, Bille J, Prod’hom G, Zanetti G, Blanc DS. Molecular epidemiology of Pseudomonas aeruginosa in intensive care units over a 10-year period (1998–2007). Clin Microbiol Infect. 2011;17(1):57–62.
Gaynes R, Edwards JR. Overview of nosocomial infections caused by Gram-negative bacilli. Clin Infect Dis. 2005;41(6):848–54.
Slama TG. Gram-negative antibiotic resistance: there is a price to pay. Crit Care. 2008. doi:10.1186/cc6820.
Crivaro V, Bogdanović L, Bagattini M, Iula VD, Catania M, Raimondi F, Triassi M, Zarrilli R. Surveillance of healthcare-associated infections in a neonatal intensive care unit in Italy during 2006–2010. BMC Infect Dis. 2015;15(1):152–9.
Xiaoyan Y, Bangrong X, Caiqian L, Zhuopeng Y, Yongbiao Z. Prevalence and fluoroquinolone resistance of Pseudomonas aeruginosa in a hospital of South China. Int J Clin Exp Med. 2015;8(1):1386–90.
Rabirad N, Mohammadpoor M, Lari AR, Shojaie A, Bayat R, Alebouyeh M. Antimicrobial susceptibility patterns of the gram-negative bacteria isolated from septicemia in Children’s Medical Center, Tehran, Iran. J Prev Med Hyg. 2014;55(1):23–6.
Vendemiato AV, von Nowakonski A, Marson FA, Levy CE. Microbiological characteristics of sepsis in a University hospital. BMC Infect Dis. 2015;15(1):58–64.
Cho HH, Kwon GC, Kim SM, Koo SH. Distribution of Pseudomonas-derived cephalosporinase and metallo-β-lactamases in carbapenem-resistant Pseudomonas aeruginosa isolates from Korea. J Microbiol Biotechnol. 2015. doi:10.4014/jmb.1503.03065.
Khan UA, Rahman H, Qasim M, Hussain A, Azizllah A, Murad W, Khan Z, Anees M, Adnan M. Alkanna tinctoria leaves extracts: a prospective remedy against multidrug resistant human pathogenic bacteria. BMC Complement Altern Med. 2015;15(1):127–32.
Khan MR, Omoloso AD. Antibacterial and antifungal activities of Dracontomelon dao. Fitoterapia. 2002;73:327–30.
Yanling Z, Shuxian L, Fen Q, Jiabo W, Yan H, Ping Z, Ruilin W, Yaming Z, Honghong L, Lifu W, Shengqiang L, Xiaohe X. Microcalorimetry coupled with principal component analysis for investigating the anti-Staphylococcus aureus effects of different extracted fractions from Dracontomelon dao. J Therm Anal Calorim. 2015;120:913–20.
Shuxian L, Yanling Z, Nan Z, Tiantian L, Yaming Z, Bin H, Jianyu L, Lifu W, Ruilin W, Man G, Yonggang L, Xiaohe X. Anti-bacterial effect of four extracts from leaves of Dracontomelon dao on Escherichia coli growth using microcalorimetry coupled with principal component analysis. J Therm Anal Calorim. 2014;116:491–7.
Yanling Z, Sisi W, Jiabo W, Ping Z, Ruisheng L, Xiaohe X. Microcalorimetry coupled with principal component analysis for comparing the effects of two Panax species on mice splenic lymphocytes. J Therm Anal Calorim. 2013;111:1669–74.
Quanfu Z, Ruisheng L, Chunyu L, Yanling Z, Ye W, Jiabo W, Ruilin W, Yaming Z, Honghong L, Jianyu L, Xiaohe X. Microcalorimetric investigation of five Aconitum L. plants on the metabolic activity of mitochondria isolated from rat liver. J Therm Anal Calorim. 2015;120:335–44.
Braissant O, Wirz D, Göpfert B, Daniels AU. Use of isothermal microcalorimetry to monitor microbial activities. FEMS Microbiol Lett. 2010;303(1):1–8.
Todinova S, Krumova S, Gartcheva L, Robeerst C, Taneva SG. Microcalorimetry of blood serum proteome: a modified interaction network in the multiple myeloma case. Anal Chem. 2011;83(20):7992–8.
Zhang T, Li X, Min X, Fang T, Zhang Z, Yang L, Liu P. Acute toxicity of chlorobenzenes in tetrahymena: estimated by microcalorimetry and mechanism. Environ Toxicol Pharmacol. 2012;33(3):377–85.
Mayeku PW, Hassanali A, Kiremire BT, Odalo JO, Hertweck C. Anti-bacterial activities and phytochemical screening of extracts of different parts of Thalictrum rhynchocarpum. Afr J Tradit Complement Altern Med. 2013;10(5):341–4.
Ingemar W. Isothermal microcalorimetry in applied biology. Thermochim Acta. 2002;394:305–11.
Cova TF, Pereira JL, Pais AA. Is standard multivariate analysis sufficient in clinical and epidemiological studies. J Biomed Inform. 2013;46:75–86.
Adam B, Tomasz B, Tomasz W, Marek S. Clinical data analysis with the use of artificial neural networks (ANN) and principal component analysis (PCA) of patients with endometrial carcinoma. Rep Pract Oncol Radiother. 2005;10(5):239–48.
Zhao Y, Wang J, Sun X, Jia L, Li J, Shan L, Li R, Liu H, Wang R, Song X, Li Y, Xiao X. Microcalorimetry coupled with chemometric techniques for toxicity evaluation of Radix Aconiti Lateralis Preparata (Fuzi) and its processed products on Escherichia coli. Appl Microbiol Biotechnol. 2014;98(1):437–44.
Gandee L, Hsieh JT, Sperandio V, Moreira CG, Lai CH, Zimmern PE. The efficacy of immediate versus delayed antibiotic administration on bacterial growth and biofilm production of selected strains of uropathogenic Escherichia coli and Pseudomonas aeruginosa. Int Braz J Urol. 2015;41(1):67–77.
Weijun K, Jiabo W, Xiaoyan X, Cheng J, Xiaohe X, Yanling Z, Ping Z, Qingce Z, Zulun L. Screening for novel antibacterial agents based on the activities of compounds on metabolism of Escherichia coli: a microcalorimetric study. J Hazard Mater. 2011;185(1):346–52.
Yanling Z, Dan Y, Jiabo W, Ping Z, Xiaohe X. Anti-fungal effect of berberine on Candida albicans by microcalorimetry with correspondence analysis. J Therm Anal Calorim. 2010;102:49–55.
Acknowledgements
The project was supported by the National Natural Science Foundation of China (81173571; 81303120), the “twelfth Five-Year Plan” Foundation of PLA (No: CWS11C164) and the major projects of the National Science and Technology (2012ZX10005010-002-002). The received support from friends was also appreciated in the course of the experiment.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, M., Qu, F., Zhao, Y. et al. Microcalorimetry and turbidimetry to investigate the anti-bacterial activities of five fractions from the leaves of Dracontomelon dao on P. aeruginosa . J Therm Anal Calorim 123, 2367–2376 (2016). https://doi.org/10.1007/s10973-015-4932-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4932-2