Abstract
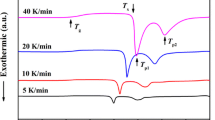
We have investigated the non-isothermal formation kinetics of nanocrystals from BaTiO3–(Li2B4O7–ZnO) (BTLBZO) glass in the temperature range 303–873 K. Thermal characteristics and structural transformations of the BTLBZO glass, formed through precursors of Li2B4O7, ZnO, and BaTiO3, have been studied by means of differential scanning calorimetry and X-ray diffraction. It is found that crystallization of glass accompanies a sequential two-step occurrence of rhombohedral ZnTiO3 and orthorhombic Ba3Zn7Ti12O34 structures. We use the non-isothermal models of the Johnson–Mehl–Avrami–Kolmogorov, Kissinger, and Ozawa equations to characterize the kinetics of the crystallization process from the BTLBZO glass. The Avrami exponents of 3.5 and 2.5, for the first and second crystallization on heating the glass, indicate that the crystallization mechanisms belong to increasing and constant nucleation rates, respectively, with the diffusion-controlled growth. The activation energies of crystallization, obtained from those non-isothermal three models, show similar values to each other, ensuring the validity of applying equations to the non-isothermal analysis of crystallization kinetics from glass. Isoconversional analysis shows that there involves a complex mechanism at the late stage of crystallization.








Similar content being viewed by others
References
Sarkar B, Chakrabarti K, Das K, De SK. Optical and ferroelectric properties of ruthenium doped BaTiO3 nanocubes. J Phys D Appl Phys. 2012;45:505304–13.
Kaygili O. Synthesis and characterization of Na2O–CaO–SiO2 glass-ceramic. J Therm Anal Calorim. 2014;117:223–7.
Liu S, Zhang H, Sviridov L, Huang L, Liu X, Samson J, Akins D, Li J, O’Brien S. Comprehensive dielectric performance of bismuth acceptor doped BaTiO3 based nanocrystal thin film capacitors. J Mater Chem. 2012;22:21862–70.
Svoboda R, Málek J. Amorphous-to-crystalline transition in Te-doped Ge2Sb2Se5 glass. J Therm Anal Calorim. 2014;117:1073–83.
Kim JE, Kim SJ, Choi HW, Yang YS. Electrical conductivity Spectra of 4BaTiO3-SiO2 Glass. J Kor Phys Soc. 2003;42:S1224–7.
Kim S, Bastani Y, Lu H, King WP, Marder S, Sandhange KH, Gruverman A, Riedo E, Bassiri-Gharb N. Direct fabrication of arbitrary-shaped ferroelectric nanostructures on plastic glass and silicon substrates. Adv Mater. 2011;23:3786–90.
Cernea M, Vasile BS, Boni A, Iuga A. Synthesis, structural characterization and dielectric properties of Nb doped BaTiO3/SiO2 core–shell heterostructure. J Alloys Compd. 2014;587:553–9.
Shao S, Zhang J, Zhang Z, Zheng P, Zhao M, Li J, Wang C. High piezoelectric properties and domain configuration in BaTiO3 ceramics obtained through the solid-state reaction route. J Phys D Appl Phys. 2008;41:125408–12.
Yang H, Zhou C, Liu X, Zhou Q, Chen G, Li W, Wang H. Piezoelectric properties and temperature stabilities of Mn- and Cu-modified BiFeO3–BaTiO3 high temperature ceramics. J Euro Ceram Soc. 2013;33:1177–83.
Fuentes S, Barraza N, Veloso E, Villarroel R. Effects of Eu substitution on luminescent and magnetic properties of BaTiO3 nanomaterials. J Alloys Compd. 2013;569:52–7.
Borah M, Mohanta D. Structural and optoelectronic properties of Eu2+-doped nanoscale barium titanates of pseudo-cubic form. J Appl Phys. 2012;112:124321–8.
Yoon SH, Park JS, Kim SH, Kim DY. Thermally stimulated depolarization current analysis for the dielectric aging of Mn and V-codoped BaTiO3 multi layer ceramic capacitor. Appl Phys Lett. 2012;103:042901–5.
Randall CA, Newnham RE, Cross LE. History of the first ferroelectric oxide. BaTiO3. Web Source. 2009 http://ceramics.org/wp-content/uploads/2009/05/first_ferroelectric_oxide_ba_tio3.pdf.
Abdel-Khalek EK, Mohamed EA, Salem SM, Ebrahim FM, Kashif I. Study of glass-nanocomposite and glass–ceramic containing ferroelectric phase. Mater Chem Phys. 2012;133:69–77.
Senyshyn A, Boysen H, Niewa R, Banys J, Kinka M, Burak Y, Adamiv V, Izumi F, Chumak I, Fuess H. High-temperature properties of lithium tetraborate Li2B4O7. J Phys D Appl Phys. 2012;45:175305–19.
Wang ZL. From nanogenerators to piezotronics—a decade-long study of ZnO nanostructures. MRS Bull. 2012;37:814–27.
Choi HW, Kim YH, Rim YH, Yang YS. Crystallization kinetics of lithium niobate glass: determination of the Johnson–Mehl–Avrami–Kolmogorov parameters. Phys Chem Chem Phys. 2013;15:9940–6.
Choi HW, Rim YH, Yang YS. Isothermal crystallization kinetics of lithium-tantalate glass: nanocrystal prepared by glass phase. J Kor Phys Soc. 2013;63:2376–80.
Avrami M. Kinetics of phase change I. J Chem Phys. 1939;7:1103–12.
Avrami M. Kinetics of phase change II. J Chem. Phys. 1940;8:212–24.
Avrami M. Kinetics of phase change III. J Chem Phys. 1941;9:177–84.
Kolmogorov AN. Statistical theory of crystallization of metals. Izvestia Akad Nauk USSR Ser Math. 1937;1:355–9.
Christian JW. The theory of transformation in metals and alloys. 2nd ed. Oxford: Pergamon; 1975.
Kissinger HE. Variation of peak temperature with heating rate in differential thermal analysis. J Res Nat Bur Stand. 1956;57:217–21.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal. 1970;2:301–24.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand A Phys Chem. 1966;70:487–523.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Akahira T, Sunose T. Joint convention of four electrical institutes. Res Rep Chiba Inst Technol. 1971;16:22–31.
Lad KN, Savalia RT, Pratap A, Dey GK, Banerjee S. Isokinetic and isoconversional study of crystallization kinetics of a Zr-based metallic glass. Thermochim Acta. 2008;473:74–80.
Venkatesh M, Ravi P, Tewari SP. Isoconversional kinetic analysis of decomposition of nitroimidazoles: friedman method vs Flynn–Wall–Ozawa Method. J Phys Chem. 2013;117:10162–9.
Mothé CG, Miranda IC. Study of kinetic parameters of thermal decomposition of bagasse and sugarcane straw using Friedman and Ozawa–Flynn–Wall isoconversional methods. J Therm Anal Calorim. 2013;113:497–505.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2010-0024388). This research was also supported by a national nuclear R&D program through the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2013M2B2A4041435).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, H.W., Yang, Y.S. Non-isothermal crystallization kinetics of BaTiO3–(Li2B4O7–ZnO) glass. J Therm Anal Calorim 119, 2171–2178 (2015). https://doi.org/10.1007/s10973-015-4391-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4391-9