Abstract
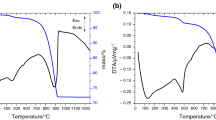
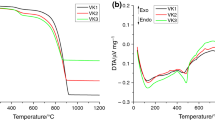
Belite or β-dicalcium silicate (β-C2S, Ca2SiO4), one of the main compounds in Portland cement clinker, is conventionally produced by sintering beyond the temperature of 1,200 °C using limestone and quartz. This study is aimed to synthesis belite from lime sludge, a residual solid waste from paper and pulp industry, with nanosilica at relatively low temperature through solid-state reaction. The precursors and synthesized final products are characterized using TG–MS–DTA, FTIR, XRD, SEM, TEM/EDAX and BET analysis. It is observed that the thermal process toward the formation of belite of mechanically activated dissimilar-sized precursors reduced the sintering temperature of the reaction significantly and belite is obtained without the use of any chemical stabilizers and calcination of lime sludge.








Similar content being viewed by others
References
Raquel VV, Moisés F, María ISR, Iñigo V, Rosario G. Mineralogical and morphological changes of calcined paper sludge at different temperatures and retention in furnace. Appl Clay Sci. 2007;36:279–86.
Da Maria DG, Joana SC. Grits and dregs for cement replacement—preliminary studies. International conference on non-conventional materials and technologies (NOCMAT 2009), Bath 2009. p. 1–8.
Dong O, Weiting X, Tommy YL, Janet FCS. Increasing mortar strength with the use of activated kaolin by-products from paper industry. Constr Build Mater. 2011;25:1537–45.
Maheswaran S, Ramesh KV, Bhuvaneshwari B, Palani GS, Nagesh RI. Studies on lime sludge for partial replacement of cement. Appl Mech Mater. 2011;71–78:1015–9. doi:10.4028/www.scientific.net/AMM.71-78.1015.
Rodríguez O, Frías M, de Rojas MIS. Influence of the calcined paper sludge on the development of hydration heat in blended cement mortars. J Therm Anal Calorim. 2008;92:865–71.
Morsy MS, Al-Salloum Y, Almusallam T, Abbas H. Effect of nano-metakaolin addition on the hydration characteristics of fly ash blended cement mortar. J Therm Anal Calorim. 2013;. doi:10.1007/s10973-013-3512-6.
Smith DK, Majumdar AJ, Ordway F. Re-examination of the polymorphism of dicalcium silicate. J Am Ceram Soc. 1961;44:405–11.
El-Didamony H, Khalil K, Ahmed TA, Heikal M. Preparation of β-dicalcium silicate (β-C2S) and calcium sulfoaluminate (C3A3CS) phases using non-traditional nano-materials. Constr Build Mater. 2012;35:77–83.
Guerrero A, Goñi S, Campillo I, Moragues A. Belite cement clinker from coal fly ash of high Ca content- optimization of synthesis parameters. Environ Sci Technol. 2004;38:3209–13.
Singh NB. Hydrothermal synthesis of β-dicalcium silicate (β-Ca2SiO4). Prog Cryst Growth Charact. 2006;52:77–83.
Pimraksa K, Hanjitsuwan S, Chindaprasirt P. Synthesis of belite cement from lignite fly ash. Ceram Int. 2009;35:2415–25.
Tevulová N, Bálintová M, Bbrianèin J, Széghyová Z. Mechnochemical synthesis of belite cements from coal fly ash/Portlandite mixture. Chem Sustain Dev. 2007;15:225–9.
Rodrigues FA. Low-temperature synthesis of cements from rice hull ash. Cem Concr Res. 2003;33:1525–9.
Nettleship I, Shull JL Jr, Kriven WM. Chemical preparation and phase stability of Ca2SiO4 and Sr2SiO4 powders. J Eur Ceram Soc. 1993;11:291–8.
Chrysa R, Perraki T, Kakali G. Sol–gel preparation of 2CaO·SiO2. J Eur Ceram Soc. 2007;27:1707–10.
Goñi S, Guerrero A. Study of alkaline hydrothermal activation of belite cements by thermal analysis. J Therm Anal Calorim. 2010;99:471–7.
Meiszterics A, Rosta L, Peterlik H, Rohonczy J, Kubuki S, Henits P, Sinkó K. Structural characterization of gel-derived calcium silicate systems. J Phys Chem A. 2010;114:10403–11.
Fernández-Carrasco L, Torrens-Martín D, Morales LM, Sagrario Martínez-Ramírez. Infrared spectroscopy in the analysis of building and construction materials. In: Theophanides T editors. Infrared spectroscopy—materials science, engineering and technology. 2012. ISBN: 978-953-51-0537-4, InTech. doi: 10.5772/36186.
Mollah MYA, Yu W, Schennach R, Cocke DL. A Fourier transform infrared spectroscopic investigation of the early hydration of Portland cement and the influence of sodium lignosulfonate. Cem Concr Res. 2000;30:267–73.
Kurdowski W, Duszak S, Trybalska B. Belite produced by means of low temperature synthesis. Cem Concr Res. 1997;27:51–61.
Whitfield PS, Mitchell LD. Quantitative rietveld analysis of the amorphous content in cements and clinkers. J Mater Sci. 2003;38:4415–21.
Jost KH, Ziemer B, Seydel R. Redetermination of the structure of β-dicalcium silicate. Acta Cryst B. 1977;33:1696–700.
Mumme WG, Hill RJ, Bushnell-Wye G, Segnit ER. Rietveld structure refinement, crystal chemistry and calculated powder diffraction data for the polymorphs of dicalcium silicate and related phases. Neues Jahrb Mineral Abh. 1995;169:35–68.
Toraya H, Yamazaki S. Simulated annealing structure solution of a new phase of dicalcium silicate Ca2SiO4 and the mechanism of structural changes from α-dicalcium silicate hydrate to αL′-dicalcium silicate via the new phase. Acta Cryst B. 2002;58:613–21.
Acknowledgements
The scientists and staff members of CSMG of CSIR-SERC are greatly acknowledged for the useful discussions and suggestions provided during the course of the investigations. This paper is being published with the kind permission of the Director, CSIR-SERC, Chennai, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maheswaran, S., Kalaiselvam, S., Arunbalaji, S. et al. Low-temperature preparation of belite from lime sludge and nanosilica through solid-state reaction. J Therm Anal Calorim 119, 1845–1852 (2015). https://doi.org/10.1007/s10973-014-4371-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4371-5