Abstract
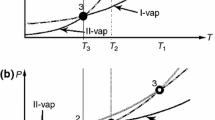
m-Anisic acid is generally recognized as a safe (GRAS) flavouring substance. Its GRAS status and the functional groups present in the molecule make it an interesting candidate for pharmaceutical co-crystallization studies. The knowledge of m-anisic acid crystalline structure/polymorphic behaviour is important information for its applications. In this work, a crystalline structure of m-anisic acid form I, T fus = 105 °C, was solved by X-ray diffraction: monoclinic, space group P21/n, with a = 13.8075(5) Å, b = 5.0221(2) Å, c = 21.4455(8) Å, β = 99.325(3)°, Mr = 331.37, V = 1467.44(10) Å3, Z = 8 and R = 0.0395 (CCDC No. 985352). A recent paper by Raffo et al. (J Mol Struct 1070:86–93, 2014) (CCDC No. 985637) also resolved the structure, and within experimental error, the two structures are equal. The molecular flexibility of m-anisic acid results in the presence of two conformers in the unit cell, a rare case of conformational isomorphism. These two conformers were found by ab initio calculations to differ in energy by 4.9 kJ mol−1. Solid samples were generated by crystallization from solutions and by melt cooling. Using a multidisciplinary approach involving thermal analysis (DSC, PLTM), infrared spectroscopy and X-ray powder diffraction, a new monotropic solid form II, T fus = 94 °C, was identified and characterized. Polymorph II slowly transforms into polymorph I at room temperature.












Similar content being viewed by others
References
Scientific opinion on flavouring group evaluation 96 (FGE.96). EFSA J. 2011;9(12):1924:1–60.
Food Agriculture Organization. Evaluation of certain food additives and contaminants—WHO Technical Report Series 909. Switzerland: World Health Organization; 2002.
Gunn ET, Bonner P, Santora D, inventors. Skin care composition, useful e.g. for providing moisture to mammalian skin, comprises oil, water, emulsifier and preservative comprising organic acid e.g. benzoic acid, p-anisic acid, sorbic acid, lactic acid, acetic acid or formic acid. USA patent US2011152384-A1. 2011.
Peters AF, inventor Pk Peters Krizman Sa, assignee. Composition useful as medicine for preventing and/or treating infection, preferably vaginal infection, bacterial infection and/or fungal infection, comprises anisic acid or its derivative and/or salt, and acid buffer. EU patent EP2314283-A1. 2011.
Capelli C, inventor Capelli Cristina, assignee. Antiseptic agent composition, use and preparation thereof, and formulation containing the same are disclosed. Japão patent JP2010270083-A. 2010.
Papageorgiou S, Varvaresou A, Tsirivas E, Demetzos C. New alternatives to cosmetics preservation. J Cosmet Sci. 2010;61(2):107–23.
Perlovich GL, Volkova TV, Manin AN, Bauer-Brandl A. Extent and mechanism of solvation and partitioning of isomers of substituted benzoic acids: a thermodynamic study in the solid state and in solution. J Pharm Sci. 2008;97(9):3883–96. doi:10.1002/jps.21260.
Callanan JE, Sullivan SA, Vecchia DF. Standards development for differential scanning calorimetry. J Res Natl Bur Stand. 1986;91(3):123–9.
Parvez M. Structure of o-anisic acid. Acta Crystallogr, Sect C. 1987;43(11):2243–5. doi:10.1107/S0108270187088231.
Fausto R, Matos-Beja A, Paixao JA. Molecular structure and charge density analysis of p-methoxybenzoic acid (anisic acid). J Mol Struct. 1997;435(3):207–18. doi:10.1016/s0022-2860(97)00187-7.
Raffo PA, Rossi L, Alborés P, Baggio RF. Cukiernik FD (2014) Alkoxy-benzoic acids: some lacking structures and rationalization of the molecular features governing their crystalline architectures. J Mol Struct. 2014;1070(0):86–93. doi:10.1016/j.molstruc.2014.04.003.
Lohani S, Grant DJW. Thermodynamics of polymorphs. In: Hilfiker R, editor. Polymorphism: in the pharmaceutical industry. Weiheim: Wiley VCH; 2006. p. 21–42.
McCrone WC. Polymorphism. In: Fox D, Labes MM, Weissberger A, editors. Physics and chemistry of the organic solid state. New York: Wiley Interscience; 1965. p. 725–67.
Bilton C, Howard JAK, Madhavi NNL, Nangia A, Desiraju GR, Allen FH, et al. When is a polymorph not a polymorph? Helical trimeric O–H center dot center dot center dot O synthons in trans-1,4-diethynylcyclohexane-1,4-diol. Chem Commun. 1999;17:1675–6. doi:10.1039/a905025f.
Esteves de Castro RA, Canotilho J, Barbosa RM, Silva MR, Beja AM, Paixa JA, et al. Conformational isomorphism of organic crystals: racemic and homochiral atenolol. Cryst Growth Des. 2007;7(3):496–500. doi:10.1021/cg0601857.
Maria TMR, Castro RAE, Bebiano SS, Silva MR, Beja AM, Canotilho J, et al. Polymorphism of trans-1,4-cyclohexanediol: conformational isomorphism. Cryst Growth Des. 2010;10(3):1194–200. doi:10.1021/cg901160v.
Newberne P, Smith RL, Doull J, Feron VJ, Goodman JI, Munro IC, et al. GRAS flavoring substances 19. Food Technology. 2000;54(6):66–84.
Sabbah R, An XW, Chickos JS, Leitao MLP, Roux MV, Torres LA. Reference materials for calorimetry and differential thermal analysis. Thermochim Acta. 1999;331(2):93–204. doi:10.1016/s0040-6031(99)00009-x.
Della Gatta G, Richardson MJ, Sarge SM, Stolen S. Standards, calibration, and guidelines in microcalorimetry—Part 2. Calibration standards for differential scanning calorimetry—(IUPAC Technical Report). Pure Appl Chem. 2006;78(7):1455–76. doi:10.1351/pac200678071455.
Bruker. APEX2 and SAINT. Madison: Bruker AXS Inc.; 2003.
Sheldrick GM. SADABS. Germany: University of Göttingen; 2003.
Sheldrick GM. A short history of SHELX. Acta Crystallogr, Sect A. 2008;64:112–22. doi:10.1107/s0108767307043930.
A. GA. PC GAMESS/Firefly version 7.1.G. 2009. http://classic.chem.msu.su/gran/gamess/index.html.
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, et al. General atomic and molecular electronic-structure system. J Comput Chem. 1993;14(11):1347–63. doi:10.1002/jcc.540141112.
Becke AD. Density functional exchange energy approximation with correct asymptotic behavior. Phys Rev A. 1988;38(6):3098–100. doi:10.1103/PhysRevA.38.3098.
Becke AD. Density functional thermochemistry. 3. The role of exact exchange. J Chem Phys. 1993;98(7):5648–52. doi:10.1063/1.464913.
Lee CT, Yang WT, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron-density. Phys Rev B. 1988;37(2):785–9. doi:10.1103/PhysRevB.37.785.
Steiner T. Frequency of Z’ values in organic and organometallic crystal structures. Acta Crystallogr, Sect B. 2000;56:673–6. doi:10.1107/s0108768100002652.
Desiraju GR. On the presence of multiple molecules in the crystal asymmetric unit (Z[prime or minute] > 1). Chem Eng Commun. 2007;9(1):91–2. doi:10.1039/B614933B.
Anderson KM, Steed JW. Comment on “On the presence of multiple molecules in the crystal asymmetric unit (Z[prime or minute] > 1)” by Gautam R. Desiraju, CrystEngComm, 2007, 9, 91. Chem Eng Commun. 2007;9(4):328–30. doi:10.1039/B701009E.
Etter MC. Encoding and decoding hydrogen bond patterns of organic compounds. Acc Chem Res. 1990;23(4):120–6. doi:10.1021/ar00172a005.
Bernstein J, Davis RE, Shimoni L, Chang NL. Patterns in hydrogen bonding—functionality and graph set analysis in crystals. Angew Chem Int Ed. 1995;34(15):1555–73. doi:10.1002/anie.199515551.
McKinnon JJ, Mitchell AS, Spackman MA. Hirshfeld surfaces: a new tool for visualising and exploring molecular crystals. Chem Eur J. 1998;4(11):2136–41. doi:10.1002/(sici)1521-3765(19981102)4:11<2136:aid-chem2136>3.0.co;2-g.
McKinnon JJ, Spackman MA, Mitchell AS. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr, Sect B. 2004;60:627–68. doi:10.1107/s0108768104020300.
Wolff SK, Griwood DJ, McKinnon JJ, Turner MJ, Jayatilaka D, Spackman MA. CrystalExplorer (version 3.1). Perth: University of Western Australia; 2012.
Buttar D, Charlton MH, Docherty R, Starbuck J. Theoretical investigations of conformational aspects of polymorphism. Part 1: O-acetamidobenzamide. J Chem Soc Perkin Trans. 2. 1998;763–72. doi:10.1039/a706978b.
Desiraju GR, Steiner T. The weak hydrogen bond. Oxford: Oxford University Press; 1999.
Mullin JW. Crystallization. 4th ed. Oxford: Butterworth-Heinemann; 2001.
Young RA. The Rietveld method. Oxford: Oxford University Press; 1993.
Rodriguezcarvajal J. Recent advances in magnetic-structure determination by neutron powder diffraction. Phys B. 1993;192(1–2):55–69. doi:10.1016/0921-4526(93)90108-i.
Kalinowska M, Swislocka R, Rzaczynska Z, Sienkiewicz J, Lewandowski W. Spectroscopic (FT-IR, FT-Raman, UV, (1)H, and (13)C NMR) and theoretical studies of m-anisic acid and lithium, sodium, potassium, rubidium, and caesium m-anisates. J Phys Org Chem. 2010;23(1):37–47. doi:10.1002/poc.1581.
Varsányi G. Assignments for vibrational spectra of 700 benzene derivatives. Budapest: Akadémiai Kiadó; 1973.
Iogansen AV. Direct proportionality of the hydrogen bonding energy and the intensification of the stretching v(XH) vibration in infrared spectra. Spectrochim Acta, Part A. 1999;55(7–8):1585–612. doi:10.1016/s1386-1425(98)00348-5.
Stolov AA, Borisover MD, Solomonov BN. Hydrogen bonding in pure base media. Correlations between calorimetric and infrared spectroscopic data. J Phys Org Chem. 1996;9(5):241–51. doi:10.1002/(sici)1099-1395(199605)9:5<241:aid-poc782>3.0.co;2-c.
Rozenberg MS. IR spectra and hydrogen bond energies of crystalline acid salts of carboxylic acids. Spectrochim Acta, Part A. 1996;52(11):1559–63. doi:10.1016/0584-8539(96)01703-5.
Foresman JB, Frisch A. Exploring chemistry with electronic structure methods. 2nd ed. Pittsburgh: Gaussian, Inc.; 1996.
Burger A, Ramberger R. On the polymorphism of pharmaceuticals and other molecular crystals. I. Mikrochim Acta. 1979;II:259–71.
Burger A, Ramberger R. On the polymorphism of pharmaceuticals and other molecular crystals. II. Mikrochim Acta. 1979;II:273–316.
Maria TR, Castro RE, Silva MR, Ramos ML, Justino LG, Burrows H, et al. Polymorphism and melt crystallisation of racemic betaxolol, a β-adrenergic antagonist drug. J Therm Anal Calorim. 2013;111(3):2171–8. doi:10.1007/s10973-012-2765-9.
Arranja C, Marcos M, Silva M, Eusébio ME, Castro RE, Sobral AFN. Synthesis and polymorphism evaluation of the 3,5-bis(decyloxy)benzaldehyde. J Therm Anal Calorim. 2014;117(3):1375–83. doi:10.1007/s10973-014-3904-2.
Gálico DA, Perpétuo GL, Castro RAE, Treu-Filho O, Legendre AO, Galhiane MS, et al. Thermoanalytical study of nimesulide and their recrystallization products obtained from solutions of several alcohols. J Therm Anal Calorim. 2014;115(3):2385–90. doi:10.1007/s10973-013-3294-x.
Abu Bakar M, Nagy Z, Rielly C. A combined approach of differential scanning calorimetry and hot-stage microscopy with image analysis in the investigation of sulfathiazole polymorphism. J Therm Anal Calorim. 2010;99(2):609–19. doi:10.1007/s10973-009-0001-z.
Acknowledgements
P. S. Pereira Silva acknowledges the support by Fundação para a Ciência e a Tecnologia, under the scholarship SFRH/BPD/84173/2012. The Center for Pharmaceutical Studies (CEF), the Coimbra Chemistry Centre (CCC) and Centro de Estudos de Materiais por Difracção de Raios-X (CEMDRX) are supported by the Fundação para a Ciência e a Tecnologia (FCT), Portuguese Agency for Scientific Research, through the projects PEst-OE/SAU/UI0177/2014, PEst-OE/QUI/UI0313/2014 and PEst-C/FIS/UI0036/2011, respectively.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Pereira Silva, P.S., Castro, R.A.E., Melro, E. et al. Structural evidence of polymorphism and conformational isomorphism of a somewhat flexible molecule: m-anisic acid. J Therm Anal Calorim 120, 667–677 (2015). https://doi.org/10.1007/s10973-014-4289-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4289-y