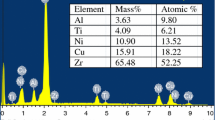
Abstract
The non-isothermal crystallization kinetics of amorphous Co69Fe3Si18B10 metallic glass has been studied using various thermal analytical models. The DSC curve of amorphous sample in a constant heating rate experiment showed the appearance of two crystallization events at peak temperatures 761 and 803 K, respectively. The activation energy for the secondary crystallization process was found to be lower compared to that of primary process which indicated the ease of secondary crystallization process from modified matrix due to the evolution of the primary phase. The activation energy remained constant during the primary crystallization process over the range of crystallization fraction (0.1–0.9), however, the local Avrami exponent for this crystallization process manifested complex variation with crystallized volume fraction. The validity of the JMA model for the primary crystallization process has been studied. This crystallization process was found to show autocatalytic behavior which has been given a mathematical description using a two-parameter model of Sestak–Berggren.













Similar content being viewed by others
References
Phan MH, Peng HX. Giant magnetoimpedance materials: fundamentals and applications. Prog Mater Sci. 2008;53:323–420.
Ozawa T. Kinetics of non-isothermal crystallisation. Polymer. 1971;12:150–8.
Henderson DW. Thermal analysis of non-isothermal crystallization kinetics in glass forming liquids. J Non-Cryst Solids. 1979;30:301–15.
Henderson DW. Experimental analysis of non-isothermal transformations involving nucleation and growth. J Therm Anal. 1979;15:325–31.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Wu J, Ye P, Pi J. On non-isothermal kinetics of two Cu-based bulk metallic glasses. J Therm Anal Calorim. 2013. doi:10.1007/s10973-013-3288-8.
Lad KN, Savalia RT, Pratap A, Dey GK, Banerjee S. Isokinetic and isoconversional study of crystallization kinetics of a Zr-based metallic glass. Thermochim Acta. 2008;473:74–80.
Lu XC, Li YH. Kinetics of non-isothermal crystallization in Cu50Zr43Al7 and (Cu50Zr43Al7)95Be5 metallic glasses. J Therm Anal Calorim. 2014;. doi:10.1007/s10973-013-3364-0.
Yinnon H, Uhlmann DR. Application of thermoanalytical techniques to the study of crystallization kinetics in glass forming liquids, Part I. Theory. J Non-Cryst Solids. 1983;54:253–62.
Altuzar P, Valenzuela R. Avrami and Kissinger theories for crystallization of metallic amorphous alloys. Mater Lett. 1991;11:101–4.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand A. 1966;70:487–523.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1970;38:1881–6.
Doyle CD. Estimating isothermal life from thermogravimetric data. J Appl Polym Sci. 1962;6:639–42.
Doyle CD. Series approximations to the equation of thermogravimetric data. Nature. 1965;207:290–1.
Shao-Xu Wang, Shi-Guang Quan, Chuang Dong. Kinetic of glass transition of Zr57.2Al21.4Ni21.4 bulk metallic glass. Thermochim Acta. 2012;532:92–5.
Patel AT, Pratap A. Kinetics of crystallization of Zr52Cu18Ni14Al10Ti6 metallic glass. J Therm Anal Calorim. 2013;. doi:10.1007/s10973-011-1549-y.
Trujillo M, Orozco A, Casas-Ruiz M, Ligero RA, Jimenez-Garay R. Crystallization kinetics study of Fe-B-Si metallic glasses in the theoretical frame of the JMA model. Mater Lett. 1995;24:287–90.
Gao YQ, Wang W. On the activation energy of crystallization in metallic glasses. J Non-Cryst Solids. 1986;81:129–34.
Nakamura K, Watanabe K, Katayama K, Amano T. Some aspects of non-isothermal crystallization of polymers. I Relationship between crystallization temperature, crystallinity and cooling conditions. J Appl Polym Sci. 1972;16:1077–91.
Nakamura K, Katayama K, Amano T. Some aspects of nonisothermal crystallization of polymers. II. Consideration of the isokinetic condition. J Appl Polym Sci. 1973;17:1031–41.
Blazquez JS, Conde CF, Conde A. Non-isothermal approach to isokinetic crystallization processes: application to the nanocrystallization of HITPERM alloys. Acta Mater. 2005;53:2305–11.
Malek J. Kinectic analysis of crystallization processes in amorphous materials. Thermochim Acta. 2000;355:239–53.
Sestak J, Berggren G. Study of kinetics of the mechanism of the solidstate reactions at increasing temperature. Thermochim Acta. 1971;3:1–12.
Hoque SM, Haque AKMR, Rahman Md O, Nghi NH, Hakim MA, Akhter S. Ultra-soft magnetic properties and giant magneto-impedance of Co68Fe4.5Si12·5B15. J Non-Cryst Solids. 2011;357:2109–14.
Chun BS, Kim SD, et al. Effects of Co addition on microstructure and magnetic properties of ferromagnetic CoFeSiB alloy films. Acta Mater. 2010;58:2836–42.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srivastava, A.P., Srivastava, D., Mazumdar, B. et al. Thermoanalytical study of crystallization process in metallic glass of Co69Fe3Si18B10 . J Therm Anal Calorim 119, 1353–1361 (2015). https://doi.org/10.1007/s10973-014-4231-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4231-3