Abstract
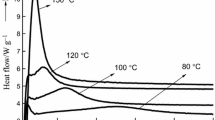
This study was undertaken to compare thermal cure kinetics of urea–formaldehyde (UF) resins, in both liquid and solid forms as a function of formaldehyde/urea (F/U) mole ratio, using multi-heating rate methods of differential scanning calorimetry. The requirement of peak temperature (T p), heat of reaction (ΔH) and activation energy (E) for the cure of four F/U mole ratio UF resins (1.6, 1.4, 1.2 and 1.0) was investigated. Both types of UF resins showed a single T p, which ranged from 75 to 118 °C for liquid resins, and from 240 to 275 °C for solid resins. As the F/U mole ratio decreased, T p values increased for both liquid and solid resins. ΔH values of solid resins were much greater than those of liquid resins, indicating a greater energy requirement for the cure of solid resins. The ΔH value of liquid UF resins increased with decreasing in F/U mole ratio whereas it was opposite for solid resins, with much variation. The activation energy (E a) values calculated by Kissinger method were greater for solid UF resins than for liquid resins. The activation energy (E α ) values calculated by isoconversional method which showed that UF resins in liquid or solid state at F/U mole ratio of 1.6 followed a multi-step reaction in their cure kinetics. These results demonstrated that thermal curing behavior of solid UF resin differed greatly from that of liquid resins, because of a greater branched network structure in the former.





Similar content being viewed by others
References
Dunky M. Urea–formaldehyde (UF) adhesive resins for wood. Int J Adhes Adhes. 1998;18:95–107.
Pizzi A. Urea–formaldehyde adhesives. In: Pizzi A, Mittal KL, editors. Handbook of adhesive technology. 2nd ed., revised and expanded, Chap 31. New York: Marcel Dekker Inc.;2003.
Myers GE. How mole ratio of UF resin affects formaldehyde emission and other properties: a literature critique. For Prod J. 1984;34(5):35–41.
Popović M, Budinski-Simendić J, Jovičić M, Mursics J, Điporović-Momčilović M, Pavličević J, Ristić I. Curing kinetics of two commercial urea–formaldehyde adhesives studied by isoconversional method. Hem Ind. 2011;65:717–26.
Dongbin F, Jianzhang L, An M. Curing characteristics of low molar ratio urea–formaldehyde resins. J Adhes Interface. 2006;7(4):45–52.
Samaržija-Janović S, Javanović V, Konstantinović S, Marković G, Marinović-Cincović M. Thermal behavior of modified urea–formaldehyde resins. J Therm Anal Calorim. 2011;104:1159–66.
Liu Z, Xiao J, Bai S, Zhang W. Study on phenomenological curing model of epoxy resin for prediction of degree of cure. J Therm Anal Calorim. 2012;109:1555–61.
Ebewele RO, Myers GE, River BH, Koutsky JA. Polyamine-modified urea–formaldehyde resins. I. Synthesis, structure, and properties. J Appl Polym Sci. 1991;47:2997–3012.
Ebewele RO. Differential scanning calorimetry and dynamic mechanical analysis of amine-modified urea–formaldehyde adhesives. J Appl Polym Sci. 1995;58:1689–700.
Park BD, Jeong HW. Effects of acid hydrolysis on microstructure of cured urea–formaldehyderesins using atomic force microscopy. J Appl Polym Sci. 2011;122:3255–62.
Park BD, Jeong HW. Hydrolytic stability and crystallinity of cured urea–formaldehyde resin adhesives with different formaldehyde/urea mole ratios. Int J Adhes Adhes. 2011;31:524–9.
Park BD, Jeong HW, Lee SM. Morphology and chemical elements detection of cured urea–formaldehyde resins. J Appl Polym Sci. 2011;120:1475–82.
Abdullah ZA, Park BD. Hydrolytic stability of cured urea–formaldehyde resins modified by additives. J Appl Polym Sci. 2009;114:1011–7.
Park BD, Kang EC, Park JY. Thermal curing behavior of modified urea–formaldehyde resin adhesives with two formaldehyde scavengers and their influence on adhesion performance. J Appl Polym Sci. 2008;110:1573–80.
Park BD, Kang EC, Park JY. Effects of formaldehyde to urea mole ratio on thermal curing behavior of urea–formaldehyde resin and properties of particleboard. J Appl Polym Sci. 2006;101:1787–92.
Park BD, Kang EC, Park JY. Differential scanning calorimetry of urea–formaldehyde adhesive resins, synthesized under different pH conditions. J Appl Polym Sci. 2006;100:422–7.
Park BD, Kim YS, Singh AP, Lim KP. Reactivity, chemical structure, and molecular mobility of urea–formaldehyde adhesives synthesized under different conditions using FTIR and solid-state 13C CP/MAS NMR spectroscopy. J Appl Polym Sci. 2003;88:2677–87.
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Park BD, Kadla JF. Thermal degradation kinetics of resole phenol–formaldehyde resin/multi-walled carbon nanotube/cellulose nanocomposite. Thermochim Acta. 2012;540:107–15.
Vyazovkin S, Sbirrazzuoli N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol Rapid Commun. 2006;27:1515–32.
Siimer K, Kaljuvee T, Christjanson P. Thermal behaviour of urea–formaldehyde resins during curing. J Therm Anal Calorim. 2003;72:607–17.
Minopoulou E, Dessipri E, Chryssikos GD, Gionis V, Paipetis A, Panayiotou C. Use of NIR for structural characterization of urea–formaldehyde resins. Int J Adhes Adhes. 2003;23:473–84.
Myers GE, Koutsky JA. Formaldehyde liberation and cure behavior of urea–formaldehyde resins. Holzforschung. 1990;44:117–26.
Szesztay M, László-Hedwvig Z, Kovacsovics E, Tüdös F. DSC application for characterization of urea/formaldehyde condensates. Holz als Roh-und Werkstoff. 1993;51:297–300.
Chuang IS, Maciel GE. NMR study of the stabilities of urea–formaldehyde resin components toward hydrolytic treatments. J Appl Polym Sci. 1994;52:1637–51.
Chuang IS. Maciel GE.13C CP/MAS NMR study of the structural dependence of urea–formaldehyde resins on formaldehyde-to-urea molar ratios at different urea concentrations and pH values. Macromolecules. 1992;25:3204–26.
Park BD, Kim JW. Dynamic mechanical analysis of urea–formaldehyde resin adhesives with different formaldehyde-to-urea molar ratios. J Appl Polym Sci. 2008;108:2045–51.
Kim MG. Examination of selected synthesis parameters for wood adhesive-type urea–formaldehyde resins by 13C NMR spectroscopy III. J Appl Polym Sci. 2001;80:2800–14.
Langmaier F, Širarová J, Mládek M, Kolomaznik K. Curing adhesives of urea–formaldehyde type with collagen hydrolysates of chrome-tanned leather waste. J Therm Anal Calorim. 2004;75:205–19.
Landqvist N. On the reaction between urea and formaldehyde in neutral and alkaline solutions. VI. Experimental studies of the activation energy and the heat of reaction. Acta Chem Scand. 1956;10:244–8.
Wisanrakkit G, Gillham JK. The glass transition temperature (Tg) as an index of chemical conversion for a high-Tg amine/epoxy system: chemical and diffusion-controlled reaction kinetics. J Appl Polym Sci. 1990;41:2885–929.
Schmidt RG, Frazier CE. Network characterization of phenol-formaldehyde thermosetting wood adhesive. Int J Adhes Adhes. 1998;18:139–46.
Nandi S, Winter HH. Swelling behavior of partially cross-linked polymers: a ternary system. Macromolecules. 2005;38:4447–55.
Nuryawan A, Park BD. Swelling properties of cured urea–formaldehyde resins of different formaldehyde to urea mole ratios. In: proceedings of Korean society of wood science and technology annual meeting, 2012. Daegu, p. 86–8.
Pratt TJ, Johns WE, Rammon RM, Plagemann WL. A novel concept on the structure of cured urea–formaldehyde resin. J Adhes. 1985;17:275–95.
Vyazovkin S. Kinetic concepts of thermally stimulated reactions in solids: a view from a historical perspective. Int Rev Phys Chem. 2000;19:45–60.
Park BD, Causin V. Crystallinity and domain size of cured urea–formaldehyde resin adhesives with different formaldehyde/urea mole ratios. Eur Polym J. 2013;49:532–7.
Park BD, Singh AP, Nuryawan A, Hwang K. MRT letter: high resolution SEM imaging of nano-architecture of cured urea–formaldehyde resin using plasma coating of osmium. Microsc Res Tech. 2013;76:1108–11.
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science and Technology (2011-0022112).This work was also supported by the Korean Ministry of Education, Science and Technology and Korean Federation of Science and Technology Societies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nuryawan, A., Park, BD. & Singh, A.P. Comparison of thermal curing behavior of liquid and solid urea–formaldehyde resins with different formaldehyde/urea mole ratios. J Therm Anal Calorim 118, 397–404 (2014). https://doi.org/10.1007/s10973-014-3946-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3946-5