Abstract
Chlorogenic acid (CGA) is present in many plants, especially in green coffee, dry plums, and bilberries. It is an important bioactive polyphenol. Studies showed that CGA has an antioxidative, bacteriostatic, anticancer, antiviral, and anti-inflammatory activity. Despite great interest in this compound, its interaction with the lipid model membrane has not yet been investigated. To better understand the relationship between the biological activity of CGA and its interaction with biological membranes, the thermotropic behavior of model lipid membranes was investigated. The effect of CGA on the model lipid membrane, specifically on the lipid bilayer phase transitions, was examined by the combined methods: differential scanning calorimetry and fluorescence spectroscopy. In particular, the degree of packing order of the hydrophilic phase of the lipid bilayer was determined using the fluorimetric method with Laurdan and Prodan probes, while the fluorescence anisotropy of the hydrophobic phase with the DPH and TMA-DPH probes. The results of the study show that CGA incorporates mainly into the hydrophilic part of membrane, changing the packing order of the polar heads of lipids. No significant changes were recorded in membrane fluidity of the hydrophobic membrane region, for the fluorescence anisotropy practically did not change. One can thus infer that CGA does not penetrate deep into the hydrophobic area of the membrane.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, plant-derived polyphenols have been intensively studied because of their various biological and pharmacological activities. Chlorogenic acid (CGA) is a phenolic compound found in various parts of the plant, in fruits, vegetables, and herbs. It is present in large quantities in coffee beans, dry plums, cocoa fruits, hawthorn, nettle, bilberries, sunflower seeds, honeysuckle, potato tubers, and apples [1–6]. The biological properties of CGA are primarily attributed to its capacity to donate hydrogen atoms of the phenolic ring to free radicals, thus inhibiting oxidation process [7]. Research confirms that CGA, among the polyphenol compounds that occur in plants, is an important natural antioxidant [5, 6, 8–12]. The polyphenols are scavengers of free radicals which are responsible for oxidizing biological structures, with resultant damage and pathological states of an organism. Epidemiological studies have shown relationships between consumption of polyphenol-rich foods and prevention of diseases, such as coronary heart disease and osteoporosis [13]. Research showed that CGA has bacteriostatic, hypoglycemic, anticancer, anti-inflammatory, and antiviral activities [3, 14–17].
The mechanisms of the wide biological activity exhibited by CGA may involve its interactions with lipid bilayers. Understanding the effects of CGA on the model lipid bilayer may help in explaining the molecular mechanism of the action of this polyphenol as an antioxidant [18–23]. The ability of polyphenols to penetrate into lipid bilayers is undoubtedly crucial to the protection against oxidation [24]. The authors [25] suggest that polyphenols that partition in the nonpolar region of the bilayer can inhibit the propagation of lipid oxidation by intercepting intramembrane radicals and/or by increasing membrane fluidity which hinders their propagation. Thus, it is important to determine the precise location of CGA in membranes and to examine its effect on membrane fluidity and packing order. Despite great interest in this compound, its interaction with the lipid model membrane has not yet been investigated.
The aim of this paper was to present a study of the interactions of CGA with lipid membranes, and specifically to determine the effect of this compound on thermotropic phase behavior of lipids and their localization in the lipid bilayer. CGA effects were studied by means of differential scanning calorimetry (DSC) and steady-state fluorimetry. The above-mentioned methods have been employed to systematically monitor the influence of CGA on different membrane regions. In particular, the degree of packing order in the hydrophilic phase of the lipid bilayer was determined by the Laurdan and Prodan fluorescence probes, while the fluorescence anisotropy of the hydrophobic phase with the DPH and TMA-DPH probes.
Differential scanning calorimetry is useful to measure the thermal properties and the effects of degradation on the structure of biodegradable polymers, and is a useful method in studies on the nature of biological systems such as proteins or lipids [26–30]. Using DSC, we monitored thermal parameters which included the temperatures of the pretransition (T p) and the main transition peak (T m), the half-width (ΔT 1/2), and change of the enthalpy (ΔH) of the main transition of lipids. We used model systems of dimyristoylphosphatidylcholines, dipalmitoylphosphatidylcholines, and dipalmitoylphosphatidylcholines with cholesterol, such as multilamellar or unilamellar liposomes, as a very good platform to elucidate the effect of CGA on some physical properties of lipid membranes [18, 19, 31–35].
Experimental
Materials
The 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC) and 1,2-dimyristoyl-sn-glycero-3-phosphatidylcholine (DMPC) lipids, cholesterol (chol), and 3-caffeoyl-d-quinic acid (CGA) (Fig. 1) were purchased from Sigma Aldrich, Steinheim, Germany. The fluorescent probes—6-dodecanoyl-2-dimethylaminonaphthalene (Laurdan), 6-propionyl-2-dimethylaminonaphthalene (Prodan), 1,6-diphenyl-1,3,5-hexatriene (DPH) and 1-(4-trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene p-toluenesulfonate (TMA-DPH)—were purchased from Molecular Probes, Eugene, Oregon, USA.
Methods
Differential scanning calorimetry (DSC)
Samples for DSC were prepared from multilamellar liposomes (MLV) of phosphatidylcholine (DPPC, DMPC) with the presence and absence of cholesterol. Pure lipid or lipid with cholesterol was dissolved in chloroform. Chloroform was very carefully evaporated to dryness under nitrogen, and thin film was formed on the flask wall. Samples were left in a vacuum pump for at least 2 h. After that, phosphate buffer of pH 7.4 and CGA were added. Next, the lipid film was washed away from the flask wall using a mechanical shaker. The solution penetrated in between the film bilayer causing its swelling and formation of multilamellar vesicles (MLV)—liposomes. The shaking was conducted in a temperature above the main phase transition of the lipid, until all the lipids were suspended as a homogenic, milky suspension. The obtained buffered suspension contained multilamellar liposomes. Final lipid concentration in the samples was 25 mg cm−3, while that of cholesterol was 2 and 10 mol%. The prepared MLV of pure lecithin or DPPC/chol (control sample) and lecithin with CGA were placed in Mettler Toledo standard aluminum crucibles with lid of 40 μl capacity. Next, the vessels were tightly closed using a special press, and incubated for 4 days at 4 °C. The measurements were made with Mettler Toledo Thermal Analysis System D.S.C. 821e, operated at the heating rate of 2 °C min−1 from 20 to 60 °C, of DPPC (from 10 to 50 °C of DMPC) and DPPC/chol. Thermal cycles were repeated three times, the experimental errors in temperature and thermal response were ±0.2 °C and ±5 %, respectively.
The data were analyzed using original software provided by Mettler Toledo in order to determine the temperature T p, T m, half-width (ΔT 1/2), and calorimetric enthalpy (ΔH) of the main phase transition.
Fluorescence spectroscopy
Samples for steady-state fluorimetry consisted of small unilamellar vesicles (SUV) with DPPC, DMPC, and DPPC with cholesterol—all modified with CGA (DPPC-CGA, DMPC-CGA, and DPPC/chol-CGA). First, the MLV were prepared similarly as for DSC measurements, and then SUV were formed by sonificating lecithin dispersion with phosphate buffer (pH 7.4) for 15 min at 20 kHz. Fluorescent probes (Laurdan, Prodan, and DPH or TMA-DPH) were added to SUV and after that samples were incubated for 15 min in darkness at room temperature. Then a solution of CGA was added, and samples were incubated for 30 min. Control samples contained lipid suspension and a suitable fluorescent probe at 1000:1 molar ratio, and appropriate compounds at 140 μM concentration were added to the remaining samples. The measurements were made at different temperatures—above and below the main phase transition of DPPC or DMPC. Thermal cycles (20–55 or 10–40 °C) were repeated three times.
The measurements were conducted with a CARY Eclipse of VARIAN fluorimeter equipped with a Peltier temperature controller DBS (temp. accuracy ± 0.1 °C). The excitation and emission wavelengths for DPH probe were λ ex = 360 nm, λ em = 425 nm, whereas for TMA-DPH probe: λ ex = 358 nm, λ em = 428 nm. The excitation wavelength for Laurdan and Prodan was 360 nm, and the emitted fluorescence was recorded at two wavelengths: 440 and 490 nm.
Fluorescence anisotropy (A) for DPH probe was calculated using the formula \( A = (I_{\text{II}} - GI_{ \bot } )/(I_{\text{II}} + GI_{ \bot } ) \), where I II and I ⊥—fluorescence intensities—were observed in directions parallel and perpendicular to the polarization direction of the exciting wave. G is an apparatus constant that depends on emission wavelength [30]. Changes in the polar group packing arrangement of the hydrophilic part of the membrane were investigated using the Laurdan and Prodan probes, on the basis of generalized polarization (GP), and were calculated using the formula \( GP = (I_{\text{g}} - I_{\text{l}} )/(I_{\text{g}} + I_{\text{l}} ) \), where I g and I l are the fluorescence intensities at the gel and fluid phase, respectively [35, 36].
Statistical analyses
Statistical analysis was carried out using Statistica 9.0 (StatSoft Inc.). All the experiments were performed at least in triplicate unless specified otherwise. Analysis of variance was carried out and significance between means was determined using Dunnett’s post hoc test. Results are presented as mean ± SD. Significant levels were defined at p < 0.05.
Results and discussion
Interactions of CGA with lipid bilayers were investigated by means of DSC and fluorescence spectroscopy. The molecular models used in our studies of the interaction of CGA with membranes are DPPC and DMPC bilayers, which are the representative phospholipids of the human erythrocyte membrane. To make the model membranes resemble the real ones as much as possible, they were modified with cholesterol. Cholesterol is essential for the normal growth and functioning of cells (e.g., by modulating the function of the membrane proteins through direct binding to sterol-specific sites on membrane proteins).
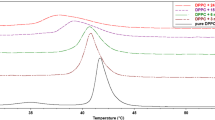
The effect of CGA on the phase transition of DPPC and DPPC/chol, determined in a DSC experiment, is shown in Fig. 2. MLV formed from DPPC showed the characteristic pretransition and the main transition. In the absence of cholesterol and CGA, DPPC liposomes display a low enthalpic pretransition at 35.4 °C and a sharp main transition at 41.4 °C (Fig. 2a). The phase transition temperatures of pure DPPC and the half-width of the main phase transition (ΔT 1/2 = 0.6 °C) are in good agreement with the literature data [37, 38]. Below the pretransition temperature (T p), the lamellar gel phase is present, in which the lipid chains are in all-trans conformation. Above the main transition temperature (T m), the fluid lamellar phase appears. In the temperature range T p −T m, an intermediate phase is observed, where bilayers are modulated by a ripple phase [38]. Cholesterol tends to fluidize the gel phase and to order the liquid-crystalline phase, thereby broadening or even eliminating the transition between the two phases (at concentration above 50 mol%). In our experiments, two different concentrations of cholesterol were used: 2 mol%, for which the pretransition could be observed, and 10 mol%, for which sharp main transition occurs [38–40].
In the studied range of concentrations, CGA practically did not affect the main phase transition temperature of MLV from DPPC (Figs. 2a, 3a, b). The peak of the main transition of DPPC is a little broadened, but remains approximately symmetric (Figs. 2a, 3b). The same effect was observed for MLV from DMPC (data not shown). These results suggest that CGA does not intercalate with the hydrophobic bilayer core. The half-width of the main phase transition (ΔT 1/2) is an index of the cooperativity of this conversion: the narrower the peak, the higher the cooperativity [29]. Compounds that interact or intercalate with the acyl chains can significantly affect the cooperativity of the transition.
In the case of MLV and the presence of cholesterol, we observed slightly greater change of T m and ΔT 1/2 than for pure lipids. It is interesting that CGA does not abolish the pretransition of DPPC but abolishes the pretransition of DPPC with 2 mol% cholesterol (Fig. 2b). These results confirm the suggestion that CGA most probably interacts with the surface of the bilayer by electrostatic interaction. Pretransition is very sensitive to any kind of perturbation, not only by compounds that penetrate the lipid bilayer but also by cosmotropic substances. Compounds that incorporate into the polar–unpolar region of the lipid bilayer abolish the DPPC pretransition even when used at low concentrations [33]. Additionally, the greater overall enthalpy suggests a lower ability of CGA to disrupt the bilayer structure (Fig. 3c). Summarizing, CGA had less influence on membranes formed from pure DPPC than on membranes modified with cholesterol. Analyzing the influence of cholesterol and investigated compound on the thermotropic parameters of membranes, it can be concluded that cholesterol (10 mol%) modifies them in greater extent than CGA, which may lead to the conclusion that CGA locates itself in the vicinity of the polar heads of phospholipids and does not disturb fluidity of the bilayer.
The phase behavior and membrane fluidity changes induced by CGA were analyzed using four probes: Laurdan, Prodan, DPH, and TMA-DPH. These fluorescent probes were used because each of them incorporates in a different region of the lipid bilayer. The TMA-DPH probe incorporates at the fourth carbon atom in the transient region between the hydrophobic and hydrophilic parts of the bilayer. The DPH probe locates in the hydrophobic, while Laurdan and Prodan—in the hydrophilic region. Such differentiated incorporation of the probes gives insight into the structural changes caused by incorporation of CGA [41, 42].
The effect of CGA on the fluidity and main phase transition of SUV formed of DPPC was studied on the basis of anisotropy measured with the DPH probe. Steady-state fluorescence anisotropy is primarily related to restriction of rotational motion of a dye in the hydrocarbon chain region. Therefore, a decrease in the anisotropy parameter can be explained by structural perturbation of the bilayer hydrophobic region due to incorporation of a compound [42]. The dependence of the DPH probe on temperature is presented in Fig. 4a. The presence of CGA practically does not change fluorescence anisotropy—we observed only slight decrease in the anisotropy of the gel-crystalline phase without changing T m. Additionally, we investigated the effect of CGA on fluidity of SUV formed of DMPC with TMA-DPH probe. While DPH is supposed to distribute in the hydrophobic bilayer, TMA-DPH is anchored at the hydrophobic–hydrophilic interface of the bilayer and fixed in the outer region of the acyl chains [43]. The fluorescence anisotropy of TMA-DPH in SUV as a function of temperature is presented in Fig. 4b. CGA practically does not change the temperature of the phase transition of DMPC, and does not change anisotropy. These results suggest that the investigated compound practically has no influence on fluidity in the hydrophobic region of the lipid bilayer, i.e., in the area of hydrocarbon chains.
Using the Laurdan and Prodan probes, we investigated the degree of order in the hydrophilic part of liposomes formed of DMPC and DPPC in the absence and presence of cholesterol. These probes are particularly sensitive to polarity of the environment and monitor relevant differences in polarities of the different phase states of lipid bilayers. Laurdan is located in the hydrophilic–hydrophobic interface of the bilayer, and its fluorophore locates near the phospholipid glycerol groups and is sensitive to polarity changes and dynamic properties at the membrane lipid–water interface [44]. The calculated values of generalized polarization (GP) of the Laurdan probe for SUV formed of DPPC and DPPC/chol lipids are presented in Fig. 5a, b. The values of GP are high and positive in the gel phase, and are much lower in the liquid-crystalline phase. CGA slightly lowers the GP coefficient both in the gel- and liquid-crystalline phases but does not change the temperature of the phase transition. Similar changes were observed for membranes containing 10 mol% cholesterol and for membranes formed from DPPC and DMPC (Fig. 6a).
Fluorescence intensity of the Prodan probe, like that of Laurdan, depends on polarity of the medium and exhibits a red shift—a fact that signifies a change of phase in the membrane and enables GP determination. Compared to Laurdan, Prodan has a different location in the lipid membrane, which is closer to the aqueous phase. Such a location allows Prodan to be also sensitive to the pretransition that occurs in the lipid polar head-groups region [44]. For that reason, we observed the occurrence of two phase transitions: a pretransition (lower-temperature transition) is connected with a change of packing and hydration of the lipid groups and the main phospholipid phase transition (higher-temperature transition). Figs. 5b and 6b allow to compare the values of GP coefficients calculated on the basis of fluorescence intensity measured at different temperatures with the Prodan probe. For liposomes formed of DPPC, large changes in GP values are observed in the gel phase. At the concentration of 140 μM, CGA does not remove the pretransition totally but changes its temperature (Fig. 5b) without changing T m. These results suggest that CGA strongly influences the gel state of the DPPC bilayer. The presence of CGA also causes a slight decrease in GP values obtained for liposomes formed of DPPC with 10 mol% cholesterol added, both in the gel and crystalline phases. Similar results were observed for SUV made of DMPC (Fig. 6b).
Conclusions
In our study, we analyzed the effect of CGA on thermotropic phase behavior of lipids and their localization in the lipid bilayer by means of DSC and fluorescence spectroscopy. We observed slight decrease of the lipid gel–liquid-crystalline phase transition temperature T m and small change of the transition enthalpy, together with a very slight broadening of the transition peaks. This suggests that CGA does not penetrate deep into the lipid bilayer and probably is located in the polar head-group region of the membrane. This conclusion is confirmed by the fluorimetric studies with DPH, TMA-DPH, Laurdan, and Prodan probes. CGA has almost no influence on fluorescence anisotropy both in the hydrophobic area and hydrophobic–hydrophilic interface of the lipid bilayer, and thus it does not change the fluidity of the bilayer in the hydrophobic area. At the same time, it slightly decreases GP, which indicates an increased disorder in the hydrophilic parts of the lipid bilayer. These effects are most pronounced in the presence of cholesterol.
In conclusion, one can infer that the protective action of CGA with respect to biological membranes depends on the extent of its incorporation in the hydrophilic part of the membrane, which is in agreement with the results we obtained for polyphenolic compounds [34].
Abbreviations
- Chol:
-
Cholesterol
- CGA:
-
Chlorogenic acid (3-caffeoyl-d-quinic acid)
- DMPC:
-
1,2-Dimirystoyl-sn-glycero-3-phosphatidylcholine
- DPPC:
-
1,2-Dipalmitoyl-sn-glycero-3-phosphatidylcholine
- DPH:
-
1,6-Diphenyl-1,3,5-hexatriene
- DSC:
-
Differential scanning calorimetry
- GP:
-
Generalized polarization
- MLV:
-
Multilamellar vesicles
- Laurdan:
-
6-Dodecanoyl-2-dimethylaminonaphthalene
- SUV:
-
Small unilamellar vesicles
- Prodan:
-
6-Propionyl-2-dimethylaminonaphthalene
- TMA-DPH:
-
1-(4-Trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene p-toluenesulfonate
References
Daayf F, Lattanzio V. Recent advances in polyphenol research, vol. 1. Oxford: Wiley-Blackwell; 2008.
Arbos KA, Claro ML, Borges L, Santos CAM, Weffort-Santos AM. Human erythrocyte as a system for evaluating the antioxidant capacity of vegetable extracts. Nutr Res. 2008;28:457–63.
Shanga X, Pana H, Li M, Miaoa X, Dingd H. Lonicera japonica Thunb.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol. 2011;138:1–21.
Bonarska-Kujawa D, Pruchnik H, Oszmiański J, Sarapuk J, Kleszczyńska H. Changes caused by fruit extracts in the lipid phase of biological and model membranes. Food Biophys. 2011;6:58–67.
Bonarska-Kujawa D, Cyboran S, Oszmiański J, Kleszczyńska H. Extracts from apple leaves and fruits as effective antioxidants. J Med Plants Res. 2011;5:2339–47.
Cyboran S, Bonarska-Kujawa D, Kapusta I, Oszmiański J, Kleszczyńska H. Antioxidant potentials of polyphenolic extracts from leaves of trees and fruit bushes. Curr Top Biophys. 2011;34:15–21.
Zang LY, Cosma G, Gardner H, Castranova V, Vallyathan V. Effect of chlorogenic acid on hydroxyl radical. Mol Cell Biochem. 2003;247:205–10.
Bouayed J, Rammal H, Dicko A, Younos C, Soulimani R. Chlorogenic acid, a from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J Neurol Sci. 2007;262:77–84.
Bonarska-Kujawa D, Sarapuk J, Bielecki K, Oszmiański J, Kleszczyńska H. Antioxidant activity of extracts from apple, chokeberry and strawberry. Pol J Food Nutr Sci. 2012;62:229–34.
Marinova EM, Toneva A, Yanishlieva N. Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem. 2009;114:1498–502.
Chu YF, Brown PH, Lyle BJ, Chen Y, Black RM, Williams CE, Lin YC, Hsu CW, Cheng IH. Roasted coffees high in lipophilic antioxidants and chlorogenic acid lactones are more neuroprotective than green coffees. J Agric Food Chem. 2009;57:9801–8.
Sato Y, Itagaki S, Kurokawa T, Ogura J, Kobayashi M, Hirano T, Sugawara M, Iseki K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int J Pharm. 2011;40:136–8.
Stocker R. Dietary and pharmacological antioxidants in atherosclerosis. Curr Opin Lipidol. 1999;10:589–97.
Yip ECH, Chan ASL, Pang H, Tam YK, Wong YH. Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c- Jun N-terminal kinase-dependent mechanism. Cell Biol Toxicol. 2006;22:293–302.
Bassoli BK, Cassolla P, Borba-Murad GR, Constantin J, Salgueiro-Pagadigorria CL, Bazotte RB, da Silva RSdSF, de Souza HM. Chlorogenic acid reduces the plasma glucose peak in the oral glucose tolerance test: effects on hepatic glucose release and glycaemia. Cell Biochem Funct. 2008;26:320–8.
Karthikesan K, Pari L, Menon VP. Protective effect of tetrahydrocurcumin and chlorogenic acid against streptozotocin–nicotinamide generated oxidative stress induced diabetes. J Funct Foods. 2010;2:134–42.
Zhao M, Wang H, Yang B, Tao H. Identification of cyclodextrin inclusion complex of chlorogenic acid and its antimicrobial activity. Food Chem. 2010;120:1138–42.
Pawlikowska-Pawlęga B, Misiak LE, Zarzyka B, Paduch R, Gawron A, Gruszecki WI. Localization and interaction of genistein with model membranes formed with dipalmitoylphosphatidylcholine (DPPC). Biochim Biophys Acta. 2012;1818:1785–93.
Arora A, Byrem TM, Nair MG, Strasburg GM. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys. 2000;373:102–9.
Sanchez-Galego JI, Lopez-Revuelat A, Sardine JL, Hernandez-Hernandez A, Sanchez-Yague J, Llianillo M. Membrane cholesterol contents modify the protective effects of quercetin and rutin on integrity and cellular viability in oxidized erythrocytes. Free Radic Biol Med. 2010;48:1444–54.
Fraga CG, Galleano M, Verstraeten SV, Oteiza PI. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol Aspects Med. 2010;31:435–45.
Hendrich AB, Michalak K. Lipids as targets for drugs modulating multidrug resistance of cancer cells. Curr Drug Targets. 2003;4:23–30.
Pawlikowska-Pawlęga B, Gruszecki WI, Misiak LE, Gawron A. The study of the quercetin action on human erythrocyte membranes. Biochem Pharmacol. 2003;66:605–12.
Fadel O, El Kirat K, Morandat S. The natural antioxidant rosmarinic acid spontaneously penetrates membranes to inhibit lipid peroxidation in situ. Biochim Biophys Acta. 2011;1808:2973–80.
Oteiza PI, Erlejman AG, Verstraeten SV, Keen CL, Fraga CG. Flavonoid–interactions: a protective role of flavonoids at the membrane surface? Clin Dev Immunol. 2005;12:19–25.
Musuc AM, Badea-Doni M, Jecu L, Rusu A, Popa VT. FTIR, XRD, and DSC analysis of the rosemary extract effect on polyethylene structure and biodegradability. J Therm Anal Calorim. 2013;114:17–169.
Yanty NAM, Marikkar JMN, Che Man YB. Effect of fractional crystallization on composition and thermal characteristics of avocado (Persea americana) butter. J Therm Anal Calorim. 2013;111:2203–9.
Ohtaka H, Kawasaki Y, Kodama M. Phase transitions of highly asymmetric chain-length N-lignocerylsphingomyelin (C24:0-SM) bilayer. J Therm Anal Calorim. 2013;113:1593–602.
Wu R-G, Wang Y-R, Wu F-G, Zhou H-W, Zhang X-H, Hou J-L. A DSC study of paeonol encapsulated liposomes, comparison the effect of cholesterol and stigmasterol on the thermotropic phase behavior of liposomes. J Therm Anal Calorim. 2012;109:311–6.
Gmajner D, Ulrih NP. Thermotropic phase behaviour of mixed liposomes of archaeal diether and conventional diester lipids. J Therm Anal Calorim. 2011;106:255–60.
Kużdżał M, Wesołowska O, Štrancar J. Fluorescence and ESR spectroscopy studies on the interaction of isoflavone genistein with biological and model membranes. Chem Phys Lipids. 2011;164:283–91.
Wesołowska O, Kużdżał M, Štrancar J, Michalak K. Interaction of the chemopreventive agent resveratrol and its metabolite, piceatannol, with model membranes. Biochim Biophys Acta. 2009;788:1851–60.
Hendrich AB, Malon R, Pola A, Shirataki Y, Motohashi N, Michalak K. Differential interaction of Sophora isoflavonoids with lipid bilayers. Eur J Pharm Sci. 2002;16:201–8.
Bonarska-Kujawa D, Pruchnik H, Kleszczyńska H. Interaction of selected anthocyanins with erythrocytes and liposome membranes. Cel Mol Biol Let. 2012;17:289–308.
Lakowicz JR. Fluorescence polarization. In: Lakowicz JR, editor. Principles of fluorescence spectroscopy. London: Plenum Press; 2006. p. 353–82.
Parasassi T, Krasnowska EK, Bagatolli L, Gratton E. Laurdan and prodan as polarity-sensitive fluorescent membrane probes. J Fluorescence. 1998;8:365–73.
Taylor KMG, Morris RM. Thermal analysis of phase transition behavior in liposomes. Therm Acta. 1995;248:289–301.
Blume A. Biological calorimetry: membranes. Therm Acta. 1991;193:299–347.
McMullen TPW, Lewis RNAH, McElhaney RN. Differential scanning calorimetric study of the effect of cholesterol on the thermotropic phase behaviour a homologous series of linear saturated phosphatidylcholines. Biochemistry. 1993;32:516–22.
McMullen TPW, McElhaney RN. New aspects of the interaction of cholesterol with dipalmitoylphosphatidylcholine bilayers as revealed by high-sensitivity differential scanning calorimetry. Biochim Biophys Acta. 1995;1234:90–8.
Harris FM, Best KB, Bell JD. Use of laurdan fluorescence intensity and polarization to distinguish between changes in membrane fluidity and phospholipid order. Biochim Biophys Acta. 2002;1565:123–8.
Dumas D, Muller S, Gouin F, Baros F, Viriot M-L, Stoltz JF. Membrane fluidity and oxygen diffusion in choresterol-enriched erythrocyte membrane. Arch Biochem Biophys. 1997;341:34–9.
Engelke M, Bojarski P, Blo R, Diel H. Tamoxifen perturbs lipid bilayer order and permeability: comparison of DSC, fluorescence anisotropy, Laurdan generalized polarization carboxyfluorescein leakage studies. Biophys Chem. 2001;200(90):157–73.
Parasassi T, De Stasio G, Ravagnan G, Rusch RM, Gratton E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys J. 1991;60:179–89.
Acknowledgements
This work was sponsored by the Ministry of Science and Education, Scientific Project No. N N312 422340 and by the Department of Physics and Biophysics of the Wrocław University of Environmental and Life Sciences—from the statutory activities fund. The use of a DSC in the Institute of Agricultural Engineering of Wrocław University of Environmental and Life Sciences is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Pruchnik, H., Bonarska-Kujawa, D. & Kleszczyńska, H. Effect of chlorogenic acid on the phase transition in phospholipid and phospholipid/cholesterol membranes. J Therm Anal Calorim 118, 943–950 (2014). https://doi.org/10.1007/s10973-014-3841-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3841-0