Abstract
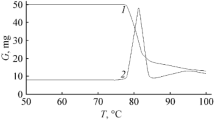
Thermal behavior and UV–Vis absorption properties of 2,5-bis(2-hydroxyphenyl)thiazolo[5,4-d]thiazole were investigated in the present study. It was found that decomposition occurs in two stages which correspond to removal of both phenolic rings and degradation of remaining core structure, respectively. After the characterization of decomposition stages, apparent activation energy values of each stage were calculated using model-free isoconversional methods (FWO and KAS). Apparent activation energies of decomposition stages are determined by both methods. Their averages are calculated as 98.232 and 123.253 kJ mol−1 in consecutive order. UV–Vis absorption properties of this compound have been determined with using different solvents.





Similar content being viewed by others
References
Ephraim J. Action of aldehydes on thioamides. Chem Ber. 1891;24:1026–31.
Johnson JR, Ketcham R, Thiazolothiazoles I. The reaction of aromatic aldehydes with dithiooxamide. J Am Chem Soc. 1960;82:2719–24.
Bevk D, Marin L, Lutsen L, Vanderzande D, Maes W. Thiazolo[5,4-d]thiazoles—promising building blocks in the synthesis of semiconductors for plastic electronics. RSC Adv. 2013;3:11418–31.
Pinto MR, Takahata Y, Atvars TDZ. Photophysical properties of 2,5-diphenyl-thiazolo[5,4-d]thiazole. J Photochem Photobiol A. 2001;143(2–3):119–27.
Bartulin J, Zuniga C, Muller H, Taylor TR. Synthesis and mesomorphic properties of 2,5-di-(4-N-alkyloxyphenyl)thiazolo[5,4-d]thiazoles. Mol Cryst Liq Cryst. 1990;180:297–304.
Helgesen M, Madsen MV, Andreasen B, Tromholt T, Andreasen JW, Krebs FC. Thermally reactive thiazolo[5,4-d]thiazole based copolymers for high photochemical stability in polymer solar cells. Polym Chem. 2011;2:2536–42.
Mamada M, Nishida J, Kumaki D, Tokito S, Yamashita Y. n-Type organic field-effect transistors with high electron mobilities based on thiazole–thiazolothiazole conjugated molecules. Chem Mater. 2007;19:5404–9.
Chaiyo N, Muanghlua R, Niemcharoen S, Boonchom B, Seeharaj P, Vittayakorn N. Non-isothermal kinetics of the thermal decomposition of sodium oxalate Na2C2O4. J Therm Anal Calorim. 2012;107:1023–9.
Su TT, Jiang H, Gong H. Thermal decomposition and dehydration kinetic studies on hydrated Co(II)methanesulfonate. Thermochim Acta. 2005;435:1–5.
Vecchio S, Materazzi S, Kurdziel K. Thermal decomposition kinetics of palladium(II) 1-allylimidazole complexes. Int J Chem Kinet. 2005;37:667–74.
Cetişli H, Çılgı GK, Donat R. Thermal and kinetic analysis of uranium salts. Part 1. Uranium(VI) oxalate hydrates. J Therm Anal Calorim. 2012;108:1213–22.
Çılgı GK, Cetişli H, Donat R. Thermal and kinetic analysis of uranium salts. Part 2. Uranium (VI) acetate hydrates. J Therm Anal Calorim. 2012;110:127–35.
Ak M, Cilgi GK, Kuru FD, Cetisli H. Thermal decomposition kinetics of polypyrrole and its star shaped copolymer. J Therm Anal Calorim. 2013;111:1627–32.
Wang SX, Tan ZC, Li YS, Sun LX, Li Y. A kinetic analysis of thermal decomposition of polyaniline/ZrO2 composite. J Therm Anal Calorim. 2008;92(2):483–7.
Liu Y, Zhao J, Zhang H, Zhu Y, Wang Z. Thermal decomposition of basic zinc carbonate in nitrogen atmosphere. Thermochim Acta. 2004;414:121–3.
Emen FM, Ocakoglu K, Külcü N. An investigation of decomposition stages of a ruthenium polypyridyl complex by non-isothermal methods. J Therm Anal Calorim. 2012;110:799–805.
Çılgı GK, Cetişli H, Donat R. Thermal and kinetic analysis of uranium salts. Part III. Uranium(IV) oxalate hydrates. J Therm Anal Calorim. 2014;115:2007–20.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Söyleyici, S., Çılgı, G.K. Thermal and kinetic analyses of 2,5-bis(2-hydroxyphenyl)thiazolo[5,4-d]thiazole. J Therm Anal Calorim 118, 705–709 (2014). https://doi.org/10.1007/s10973-014-3796-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3796-1