Abstract
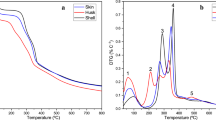
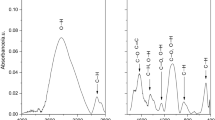
Four waste streams from a chocolate factory were examined in view of their possible usage as a fuel: cocoa shell, jute bags, and two qualities of chocolate waste (milk, white). Thus, proximate and ultimate analyses and thermogravimetric analyses coupled with Fourier transform infrared (TG-FTIR) analyses were conducted. It was observed that milk and white chocolates have similar thermal properties; chocolate has a high calorific value (24.5 MJ kg−1). Pyrolysis of chocolate proceeds in two stages: the first from 190 to 300 °C and the second from 300 to 518 °C. During the first stage, alkaloids, such as theobromine and caffeine, evolve, and sugar decomposes, releasing acids, CO2, and water. During the second pyrolysis stage, cocoa butter and proteins decompose releasing volatile organics such as esters, acids, amides, phenols, CH4, CO, etc. Polyphenols such as catechin, procyanidins, etc. decompose during both pyrolysis stages. Generally, chocolate waste yields less CO2 and CO than cocoa shell and jute. In principle, it appears to be a promising source of energy and could be utilized by both co-firing and pyrolysis, producing fuels or chemicals.





Similar content being viewed by others
References
Watson RR, Preedy VR, Zibadi S. Chocolate in health and nutrition. New York: Springer; 2012.
Jansson DS, Galgan V, Schubert B, Segerstad CA. Theobromine intoxication in a red fox and a European badger in Sweden. J Wildlife Dis. 2001;37(2):362–5.
Curtis PE, Griffiths JE. Suspected chocolate poisoning of calves. Vet Rec. 1972;90:313–4.
Alexander J, Benford D, Cockburn A, Cravedi JP, Dogliotti E, Domenico AD, et al. Opinion of the scientific panel on contaminants in the food chain on a request from the European commission to perform a scientific risk assessment on nitrate in vegetables. EFSA J. 2008;689:1–79.
Zoumas BL, Kreiser WR, Martin R. Theobromine and caffeine content of chocolate products. J Food Sci. 2006;45(2):314–6.
Commission E. Directive 2000/36/EC of the European Parliament and the Council of 23 June 2000 relating to cocoa and chocolate products intended for human consumption. Off J L. 2000;197:19–25.
Andrews R. All about chocolate. http://www.precisionnutrition.com/all-about-chocolate (2009).
Leung DY, Wu X, Leung M. A review on biodiesel production using catalyzed transesterification. Appl Energy. 2010;87(4):1083–95.
Singh SP, Singh D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: a review. Renew Sustain Energy Rev. 2010;14(1):200–16.
Haytowitz D, Lemar L, Pehrsson P, Exler J, Patterson K, Thomas R et al. USDA National Nutrient Database for standard reference, Release 24 (2012).
Cseke LJ, Kirakosyan A, Kaufman PB, Warber S, Duke JA, Brielmann HL. Natural products from plants. 2nd ed. Boca Raton: CRC Press; 2006.
Viswas A, Tewari K. A textbook of organic chemistry. 3rd ed. New Delhi: Vikas Publishing House; 2009.
Gu L, Suzanne E, Wu X, Ou B, Ronald L. Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J Agr Food Chem. 2006;54(11):4057–61.
Kealey KS, Snyder RM, Romanczyk Jr LJ, Hammerstone Jr JF, Buck MM, Cipolla GG. Foods containing cocoa solids having high cocoa polyphenol contents. Google Patents (2002).
Kirschbaum MUF. The temperature dependence of organic-matter decomposition—still a topic of debate. Soil Biol Biochem. 2006;38(9):2510–8.
Galletti GC. Py-GC-ion trap detection of sorghum grain polyphenols (syn. vegetable tannins): preliminary results. Fuel Chem Div ACS. 1991;36:691–702.
Rowland SJ. The protein distribution in normal and abnormal milk. J Dairy Res. 1938;9(01):47–57.
Hansson K, Samuelsson J, Tullin C, Mand L. Formation of HNCO, HCN, and NH3 from the pyrolysis of bark and nitrogen-containing model compounds. Combust Flame. 2004;137(3):265–77.
Crawshaw R. Co-product feeds: animal feeds from the food and drinks industries. Nottingham: Nottingham University Press; 2001.
Yi Q, Qi F, Cheng G, Zhang Y, Xiao B, Hu Z, et al. Thermogravimetric analysis of co-combustion of biomass and biochar. J Therm Anal Calorim. 2013;112(3):1475–9.
Villanueva M, Proupín J, Rodriguez-Anon J, Fraga-Grueiro L, Salgado J, Barros N. Energetic characterization of forest biomass by calorimetry and thermal analysis. J Therm Anal Calorim. 2011;104(1):61–7.
Ta S, Yürüm Y. Co-firing of biomass with coals. J Therm Anal Calorim. 2012;107(1):293–8.
Figen AK, Smail O, Pi Kin S. Devolatilization non-isothermal kinetic analysis of agricultural stalks and application of TG-FT/IR analysis. J Therm Anal Calorim. 2012;107(3):1177–89.
Arts IC, van de Putte B, Hollman PC. Catechin contents of foods commonly consumed in The Netherlands. 2. Tea, wine, fruit juices, and chocolate milk. J Agr Food Chem. 2000;48(5):1752–7.
Adamson GE, Lazarus SA, Mitchell AE, Ronald L, Cao G, Jacobs PH, et al. HPLC method for the quantification of procyanidins in cocoa and chocolate samples and correlation to total antioxidant capacity. J Agr Food Chem. 1999;47(10):4184–8.
Hammerstone JF, Lazarus SA, Mitchell AE, Rucker R, Schmitz HH. Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography/mass spectrometry. J Agr Food Chem. 1999;47(2):490–6.
Vinson JA, Proch J, Zubik L. Phenol antioxidant quantity and quality in foods: cocoa, dark chocolate, and milk chocolate. J Agr Food Chem. 1999;47(12):4821–4.
Bassilakis R, Carangelo RM, Wojtowicz MA. TG-FTIR analysis of biomass pyrolysis. Fuel. 2001;80(12):1765–86.
Fasina O, Littlefield B. TG-FTIR analysis of pecan shells thermal decomposition. Fuel Process Technol. 2012;102:61–6.
Sharma RK, Chan WG, Hajaligol MR. Effect of reaction conditions on product distribution from the co-pyrolysis of α-amino acids with glucose. J Anal Appl Pyrol. 2009;86(1):122–34.
Joardder MUH, Islam MR, Beg M, Alam R. Pyrolysis of coconut shell for bio-oil. In: International conference on Mechanical Engineering 2011, Dhaka. Bangladesh University of Engineering and Technology; 2011.
Souza BS, Moreira APD, Teixeira AMR. TG-FTIR coupling to monitor the pyrolysis products from agricultural residues. J Therm Anal Calorim. 2009;97(2):637–42.
Asadullah M, Anisur Rahman M, Mohsin Ali M, Abdul Motin M, Borhanus Sultan M, Robiul Alam M, et al. Jute stick pyrolysis for bio-oil production in fluidized bed reactor. Bioresour Technol. 2008;99(1):44–50.
Tao L, Zhao G, Qian J, Qin Y. TG–FTIR characterization of pyrolysis of waste mixtures of paint and tar slag. J Hazard Mater. 2010;175(1):754–61.
Parikh J, Channiwala SA, Ghosal GK. A correlation for calculating HHV from proximate analysis of solid fuels. Fuel. 2005;84(5):487–94.
Arenillas A, Rubiera F, Arias B, Pis JJ, Faundez JM, Gordon AL, et al. A TG/DTA study on the effect of coal blending on ignition behaviour. J Therm Anal Calorim. 2004;76(2):603–14.
Vassilev SV, Baxter D, Vassileva CG. An overview of the behaviour of biomass during combustion: part I. Phase-mineral transformations of organic and inorganic matter. Fuel. 2013;112:391–449.
Jiang X, Feng Y, Lv G, Du Y, Qin D, Li X, et al. Bioferment residue: TG-FTIR study and cocombustion in a MSW incineration plant. Environ Sci Technol. 2012;46(24):13539–44.
De Jong W, Di Nola G, Venneker B, Spliethoff H, Wójtowicz MA. TG-FTIR pyrolysis of coal and secondary biomass fuels: determination of pyrolysis kinetic parameters for main species and NO x precursors. Fuel. 2007;86(15):2367–76.
Biagini E, Barontini F, Tognotti L. Devolatilization of biomass fuels and biomass components studied by TG/FTIR technique. Ind Eng Chem Res. 2006;45(13):4486–93.
Idris SS, Rahman NA, Ismail K, Alias AB, Rashid ZA, Aris MJ. Investigation on thermochemical behaviour of low rank Malaysian coal, oil palm biomass and their blends during pyrolysis via thermogravimetric analysis (TGA). Bioresour Technol. 2010;101(12):4584–92.
Song C, Hu H, Zhu S, Wang G, Chen G. Nonisothermal catalytic liquefaction of corn stalk in subcritical and supercritical water. Energy Fuel. 2004;18(1):90–6.
Yang H, Yan R, Chen H, Lee DH, Zheng C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel. 2007;86(12):1781–8.
Carrier M, Loppinet-Serani A, Denux D, Lasnier J, Ham-Pichavant F, Cansell F, et al. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy. 2011;35(1):298–307.
Basak RK, Saha SG, Sarkar AK, Saha M, Das NN, Mukherjee AK. Thermal properties of jute constituents and flame retardant jute fabrics. Text Res J. 1993;63(11):658–66.
Redgwell R, Trovato V, Merinat S, Curti D, Hediger S, Manez A. Dietary fibre in cocoa shell: characterisation of component polysaccharides. Food Chem. 2003;81(1):103–12.
Cao J, Xiao G, Xu X, Shen D, Jin B. Study on carbonization of lignin by TG-FTIR and high-temperature carbonization reactor. Fuel Process Technol. 2012;106:41–7.
Quan C, Li A, Gao N. Research on pyrolysis of PCB waste with TG-FTIR and Py-GC/MS. J Therm Anal Calorim. 2012;110(3):1463–70.
Sami M, Annamalai K, Wooldridge M. Co-firing of coal and biomass fuel blends. Prog Energy Combust. 2001;27(2):171–214.
Biagini E, Lippi F, Petarca L, Tognotti L. Devolatilization rate of biomasses and coal-biomass blends: an experimental investigation. Fuel. 2002;81(8):1041–50.
Di Nola G, De Jong W, Spliethoff H. TG-FTIR characterization of coal and biomass single fuels and blends under slow heating rate conditions: partitioning of the fuel-bound nitrogen. Fuel Process Technol. 2010;91(1):103–15.
Pricewaterhouse. Disposal protocol. In: Jute ecolabel. http://www.jute.com:8080/c/document_library (2006).
Suzuki T, Yamada T, Okazaki N, Tada A, Nakanishi M, Futamata M, et al. Electromagnetic shielding capacity of wood char loaded with nickel. Mater Sci Res Int. 2001;7(3):206–12.
Ross AB, Jones JM, Kubacki ML, Bridgeman T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour Technol. 2008;99(14):6494–504.
Acknowledgements
The project was supported by the National Basic Research Program (973 Program) of China (2011CB201500), the Research Project of environmental protection commonwealth industry (201209023-4), the National High Technology Research and Development Program of China (2009AA064704), the National High Technology Research and Development Program of China (863 Program, 2012AA063505), Zhejiang University President Special Fund (585100-172210331), the Program of Introducing Talents of Discipline to University (B08026).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Du, Y., Jiang, X., Lv, G. et al. TG-pyrolysis and FTIR analysis of chocolate and biomass waste. J Therm Anal Calorim 117, 343–353 (2014). https://doi.org/10.1007/s10973-014-3741-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3741-3