Abstract
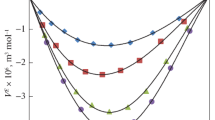
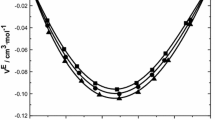
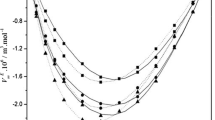
Density (ρ), viscosity (η), and speed of sound (U) values for the binary mixture systems of methyl benzoate + 2-propanol and ethyl benzoate + 2-propanol including those of pure liquids were measured over the entire mole fraction range at five different temperatures (303.15, 308.15, 313.15, 318.15, and 323.15) K. From these experimentally determined values, various thermo-acoustic parameters such as excess isentropic compressibility \( \left( {K_{\text{s}}^{\text{E}} } \right) \), excess molar volume (V E) and excess free length \( \left( {L_{\text{f}}^{\text{E}} } \right) \), excess Gibb’s free energy (ΔG *E), and excess enthalpy (H E) have been calculated. The excess functions have been fitted to the Redlich–Kister type polynomial equation. The deviations for excess thermo-acoustic parameters have been explained on the basis of the intermolecular interactions present in these binary mixtures. The theoretical values of speed of sound in the mixtures have been evaluated using various theories and have been compared with experimentally determined speed of sound values in order to check the applicability of such theories to the liquid mixture systems under study. Viscosity data have been used to test the applicability of standard viscosity models of Grunberg–Nissan, Hind–Mc Laughlin, Katti–Chaudhary, Heric and Brewer, Frenkel, Tamura and Kurata at various temperatures for the binary liquid systems under study.





Similar content being viewed by others
References
Oswal SL, Oswal P, Shalak RP. Speed of sound, isentropic compressibilities and excess molar volumes of binary mixtures containing p-dioxane. J Solut Chem. 1998;27:507–20.
Kumar H, Kaur M, Gaba R, Kaur K. Thermodynamics of binary liquid mixtures of cyclopentane with 2-propanol, 1-butanol and 2-butanol at different temperatures. J Therm Anal Calorim. 2011;105:1071–80.
Zorebski E, Waligora A. Densities, excess molar volumes, and isobaric thermal expansibilities for 1,2-ethanediol + 1-butanol, or 1-hexanol, or 1-octanol in the temperature range from (293.15 to 313.15) K. J Chem Eng Data. 2008;53:591–5.
Boruń A, Żurada M, Bald A. Densities and excess molar volumes for mixtures of methanol with other alcohols at temperatures (288.15–313.15 K). J Therm Anal Calorim. 2010;100:707–15.
Dash SK, Pradhan SK, Dalai B, Moharana L, Swain BB. Studies on molecular interaction in binary mixtures of diethyl ether with some alkanols—an acoustic approach. Phys Chem Liq. 2012;50:735–49.
Checoni RF. Excess molar enthalpy for methanol, ethanol, 1-propanol, 1-butanol + n-butylamine mixtures at 288.15 and 308.15 K at atmospheric pressure. J Therm Anal Calorim. 2010;101:349–57.
Sreenivasulu K, Govinda V, Venkateswarlu P, Sivakumar K. Thermodynamic properties of non-electrolyte solutions. J Therm Anal Calorim. 2013; doi: 10.1007/s10973-013-3395-6.
Savaroglu G, Aral E. Speeds of sound and isentropic compressibilities in binary mixtures of 2-propanol with several 1-alkanols at 298.15 K. Int J Thermophys. 2005;26(5):1525–35.
Sastry SS, Babu S, Vishwam T, Parvateesam K, Tiong HS. Excess parameters for binary mixtures of ethyl benzoate with 1-propanol, 1-butanol and 1-pentanol at T = 303, 308, 313, 318, and 323 K. Phys B. 2013;420:40–8.
Babu S, Sastry SVK, Tiong HS, Sastry SS. Experimental and theoretical studies of ultrasonic velocity in binary liquid mixtures of ethyl benzoate. J Chem. 2012;9(4):2309–14.
Sastry SVK, Babu S, Tiong HS, Sastry SS. Molecular interaction studies in ternary mixture of ethyl hydroxy benzoate by ultrasonic velocity measurements. Res J Pharm Biol Chem Sci. 2012;3(2):500–5.
Sastry SVK, Babu S, Tiong HS, Sastry SS. Ultrasonic investigation of molecular interactions in ternary mixtures at 303 K. J Chem Pharm Res. 2012;4(4):2122–5.
Glinski J, Chavepeyer G, Platten JK. Surface properties of diluted solutions of n-heptane, n-octanol and n-octanoic acid in nitromethane. Chem Phys. 2001;272:119–26.
Salgado DG, Tovar CA, Cerdeirina CA, Carballo E, Romani L. Second-order excess derivatives for the 1,3-dichloropropane + n-dodecane system. Fluid Phase Equilib. 2002;199:121–34.
Resa JM, Gonzalez C, Goenaga JM, Iglesias M. Influence of temperature on ultrasonic velocity measurements of ethanol + water + 1-propanol mixtures. J Therm Anal Calorim. 2007;87:237–45.
Sharma S, Jasmin B, Ramani J, Patel R. Density, excess molar volumes and refractive indices of β-pinene with o, m, p-xylene and toluene at 303.15, 308.15 and 313.15 K. Phys Chem Liq. 2011;49:765–76.
Redlich O, Kister AT. Algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem. 1948;40:345–8.
Vogel AI. Text book of organic chemistry. 5th ed. New York: Wiley; 1989.
Vasudha K, Kumari DV, Yuvaraja G, Krishnaiah A. Excess volumes and viscosities for the binary systems of 2-propanol with alkyl acetates at 303.15 K. J Chem Pharm Res. 2011;3(5):108–15.
Mutalik V, Manjeshwar LS, Sairam M, Aminabhavi TM. Excess molar volumes, deviations in viscosity and refractive index of the binary mixtures of mesitylene with ethanol, propan-1-ol, propan-2-ol, butan-1-ol, pentan-1-ol, and 3-methylbutan-1-ol at 298.15, 303.15, and 308.15 K. J Mol Liq. 2006;129:147–54.
Sreekanth K, Kondaiah M, Kumar DS, Rao DK. Influence of temperature on thermodynamic properties of acid–base liquid mixtures. J Therm Anal Calorim. 2012;110:1341–52.
Mohan TM, Sastry SS, Murthy VRK. Thermodynamic, dielectric and conformational studies on hydrogen bonded binary mixtures of propan-1-ol with methyl benzoate and ethyl benzoate. J Solut Chem. 2011;40:131–46.
Riddick JA, Bunger WB, Sakano TK. Techniques of chemistry. Organic solvents. 4th ed. New York: Wiley; 1986.
Aminabhavi TM, Phayde TSM, Khinnavar SR, Gopalakrishna B, Keith CH. Densities, refractive indices, speeds of sound, and shear viscosities of diethylene glycol dimethyl ether with ethyl acetate, methyl benzoate, ethyl benzoate, and diethyl succinate in the temperature range from 298.15 to 318.15 K. J Chem Eng Data. 1994;39:251–60.
Chueh CF, Swanson AC. Estimated group contribution method. In: Reid RC, Prausnitz JM, Poling BE. The properties of gases and liquids. 4th ed. New York: McGraw Hill; 1987. pp 138
Kiyohara O, Benson GC. Ultrasonic speeds and isentropic compressibilities of n-alkanol + n-heptane mixtures at 298.15 K. J Chem Thermodyn. 1979;11:861–73.
Benson GC, Kiyohara O. Evaluation of excess isentropic compressibilities and isochoric heat capacities. J Chem Thermodyn. 1979;11:1061–4.
Douheret G, Pal A, Davis MI. Ultrasonic speeds and isentropic functions of (a 2-alkoxyethanol + water) at 298.15 K. J Chem Thermodyn. 1990;22:99–108.
Narendra K, Srinivasu Ch, Kalpana Ch, Narayanamurthy P. Excess thermo dynamical parameters of binary mixtures of toluene and mesitylene with anisaldehyde using ultrasonic technique at different temperatures. J Therm Anal Calorim. 2012;107:25–30.
Pandey JD, Rai RD, Shukla RK, Shukla AK, Mishra N. Ultrasonic and thermodynamic properties of quaternary liquid system at 298.15 K. Indian J Pure Appl Phys. 1993;31:84–90.
Fort RJ, Moore WR. Adiabatic compressibilities in binary liquid mixtures. Trans Faraday Soc. 1965;61:2102–10.
Gupta M, Vibhu I, Shukla JP. Ultrasonic velocity, viscosity and excess properties of binary mixture of tetrahydrofuran with 1-propanol and 2-propanol. Fluid Phase Equilib. 2006;244:26–32.
Iloukhani H, Zoorasna N, Sloeimani R. Excess molar volumes and speeds of sound of tetrahydrofuran with chloroethanes or chloroethenes at 298.15 K. Phys Chem Liq. 2005;43:391–401.
Bhatia SC, Rani R, Bhatia R, Anand H. Volumetric and ultrasonic behaviour of binary mixtures of 1-nonanol with o-cresol, m-cresol, p-cresol and anisole at T = (293.15 and 313.15) K. J Chem Thermodyn. 2011;43:479–86.
García B, Aparicio S, Navarro AM, Alcalde R, Leal JM. Measurements and modeling of thermophysical behavior of (C1–C4) alkylbenzoate/(C1–C11) alkan-1-ol mixed solvents. J Phys Chem B. 2004;108:15841–50.
Narendra K, Srinivasu Ch, Fakruddin Sk, Narayanamurthy P. Excess parameters of binary mixtures of anisaldehyde with o-cresol, m-cresol and p-cresol at T = (303.15, 308.15, 313.15, and 318.15) K. J Chem Thermodyn. 2011;43:1604–11.
Oswal SL, Pandiyan V, Kumar BK, Vasantharani P. Thermodynamic and acoustic properties of binary mixtures of oxolane with aniline and substituted anilines at 303.15, 313.15 and 323.15 K. Thermochim Acta. 2010;507:27–34.
Subha MCS, Swamy GN, Bal ME, Rao KSKV. Excess volume and viscosity of ethoxy ethanol with n-butylamine, sec-butylamine, tert-butylamine, n-hexylamine, n-octylamine and cyclohexylamine. Indian J Chem A. 2004;43:1876–81.
Narendra K, Srinivasu Ch, Narayanamurthy P. Excess properties of binary mixtures of o-xylene, m-xylene and p-xylene with anisaldehyde at different temperatures. J Appl Sci. 2012;12(2):136–44.
Nomoto O. Empirical formula for sound velocity in binary liquid mixtures. J Phys Soc Jpn. 1958;13:1528–32.
Baluja S, Parrania PH. Acoustical properties of 3-α-furyl acrylic acid in protic and aprotic solvents. Asian J Chem. 1995;7:417–23.
Van Dael W, Vangeel E. Theory of ultrasound. In: Van Deal W. Thermodynamic properties and velocity of sound, London: Butterworth; 1975, Chap. 5.
Junjie Z. Junjie’s theory of ultrasound. In: Savaroglu G, Aral E. Densities, speeds of sound and isentropic compressibilities of the ternary mixture of 2-propanol + acetone + cyclohexane and the constituent binary mixtures at 298.15 K and 303.15 K. Fluid Phase Equilib. 2004;215:253–62.
Junjie Z. J. China. Univ. Sci. Technol. 1984;14:298–300.
Jacobson B. Ultrasonic velocity in liquids and liquid mixtures. J Chem Phys. 1952;20:927–8.
Rao GVR, Sarma AVV, Krishna JS, Rambabu C. Theoretical evaluation of ultrasonic velocities in binary liquid mixtures of o-chlorophenol at different temperatures. Indian J Pure Appl Phys. 2005;43:345–54.
Grunberg L, Nissan AH. Mixture law for viscosity. Nature. 1949;164:799–800.
Hind RK, Mc Laughlin E, Ubbelohde AR. Structure and viscosity of liquids camphor + pyrene mixtures. Trans Faraday Soc. 1960;56:328–30.
Katti PK, Chaudhari MM. Viscosities of binary mixtures of benzyl acetate with dioxane, aniline and m-cresol. J Chem Eng Data. 1964;9:442–3.
Heric EL, Brewer JC. On the viscosity of ternary mixtures. J Chem Eng Data. 1966;11:66–8.
Frenkel YI. Theory of the viscosity of liquid mixtures. Petroleum. 1946;9:27.
Tamura M, Kurata M. On the viscosity of binary mixture of liquids. Bull Chem Soc Jpn. 1952;25:32–8.
Mohan TM, Sastry SS, Murthy VRK. Conformational and dielectric relaxation studies on hydrogen bonded binary mixture of isopropyl alcohol in methyl benzoate and ethyl benzoate. J Mol Struct. 2010;973:157–62.
Acknowledgements
The authors gratefully acknowledge the Project No.: ERIP/ER/0703688/M/01/1134, dated 31-03-2010 of DRDO and UGC DRS LEVEL III program No. F.530/1/DRS/2009 (SAP-I), dated 09-02-2009 New Delhi, to the department of Physics, ANU for providing financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sastry, S.S., Babu, S., Vishwam, T. et al. Excess parameters for binary mixtures of alkyl benzoates with 2-propanol at different temperatures. J Therm Anal Calorim 116, 923–935 (2014). https://doi.org/10.1007/s10973-013-3570-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3570-9