Abstract
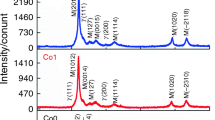
The crystallization kinetics of Cu50Zr43Al7 and (Cu50Zr43Al7)95Be5 metallic glasses was studied using differential scanning calorimetry (DSC) at four different heating rates under non-isothermal condition. The glass transition temperature T g, the onset temperature of crystallization T x, and the peak temperature of crystallization T p of the two metallic glasses were determined from DSC curves. The values of various kinetic parameters such as the activation energy of glass transition E g, activation energy of crystallization E p, Avrami exponent n and dimensionality of growth m were evaluated from the dependence of T g and T p on the heating rate. The values of E g and E p, calculated from many different models, are found to be in good agreement with each other. The average values of the Avrami exponent n are (2.8 ± 0.4) for Cu50Zr43Al7 metallic glass and (4.2 ± 0.3) for (Cu50Zr43Al7)95Be5 metallic glass, which are consistent with the mechanism of two-dimensional growth and three-dimensional growth, respectively. Finally, the parameter H r, S, and crystallization enthalpy ΔH c are introduced to estimate the glass-forming ability and thermal stability of metallic glasses. The result shows that the addition of Be improves the glass-forming ability and thermal stability of Cu50Zr43Al7 metallic glass.












Similar content being viewed by others
References
Cheung TL, Shek CH. Thermal and mechanical properties of Cu–Zr–Al bulk metallic glasses. J Alloy Compd. 2007;434–435:71–4. doi:10.1016/j.jallcom.2006.08.109.
Suryanarayana C, Inoue A. Bulk metallic glasses. Boca Raton: CRC Press; 2011.
Wang Q, Wang YM, Qiang JB, Zhang XF, Shek CH, Dong C. Composition optimization of the Cu-based Cu–Zr–Al alloys. Intermetallics. 2004;12(10–11):1229–32. doi:10.1016/j.intermet.2004.07.002.
Inoue A, Zhang W. Formation, thermal stability and mechanical properties of Cu–Zr–Al bulk glassy alloys. Mater Trans. 2002;43(11):2921–5.
Malekan M, Shabestari SG, Gholamipour R, Seyedein SH. Effect of Ge addition on mechanical properties and fracture behavior of Cu–Zr–Al bulk metallic glass. J Alloy Compd. 2009;484(1–2):708–11. doi:10.1016/j.jallcom.2009.05.023.
Qin C, Zhang W, Kimura H, Asami K, Inoue A. New Cu–Zr–Al–Nb bulk glassy alloys with high corrosion resistance. Mater Trans. 2004;45(6):1958–61.
Inoue A, Zhang T, Nishiyama N, Ohba K, Masumoto T. Preparation of 16 mm diameter rod of amorphous Zr65Al7.5Ni10Cu17.5 alloy. Mater Trans-JIM. 1993;34:1234.
Sung DS, Kwon OJ, Fleury E, Kim KB, Lee JC, Kim DH, et al. Enhancement of the glass forming ability of Cu–Zr–Al alloys by Ag addition. Met Mater Int. 2004;10(6):575–9. doi:10.1007/BF03027421.
Xu D, Duan G, Johnson WL. Unusual glass-forming ability of bulk amorphous alloys based on ordinary metal copper. Phys Rev Lett. 2004;92(24):245504.
Kim KH, Lee SW, Ahn JP, Fleury E, Kim YC, Lee JC. A Cu-based amorphous alloy with a simultaneous improvement in its glass forming ability and plasticity. Met Mater Int. 2007;13(1):21–4. doi:10.1007/BF03027818.
Lee SW, Lee SC, Kim YC, Fleury E, Lee JC. Design of a bulk amorphous alloy containing Cu–Zr with simultaneous improvement in glass-forming ability and plasticity. J Mater Res. 2007;22(02):486–92.
Kim YC, Lee JC, Cha PR, Ahn JP, Fleury E. Enhanced glass forming ability and mechanical properties of new Cu-based bulk metallic glasses. Mater Sci Eng, A. 2006;437(2):248–53.
Xu JF, Liu F, Jian ZY, Chang FE, Zhang K, Ma YZ. Phase transformation kinetics of Ge23Se67Sb10 glass. J Non-Cryst Solids. 2010;356(41–42):2198–202. doi:10.1016/j.jnoncrysol.2010.08.018.
Patial BS, Thakur N, Tripathi SK. A non-isothermal crystallization study of Se85Te15 chalcogenide glass using differential scanning calorimetry. Phys Scripta. 2012;85(4):045603. doi:10.1088/0031-8949/85/04/045603.
Abdel-Rahim MA. Crystallization kinetics of selenium–tellerium glasses. J Mater Sci. 1992;27(7):1757–61. doi:10.1007/BF01107200.
Joshi SR, Pratap A, Saxena NS, Saksena MP, Kumar A. Heating rate and composition dependence of the glass transition temperature of a ternary chalcogenide glass. J Mater Sci Lett. 1994;13(2):77–9. doi:10.1007/BF00416803.
Rabinal MK, Sangunni KS, Gopal ESR. Chemical ordering in Ge20Se80−χInχ glasses. J Non-Cryst Solids. 1995;188(1–2):98–106. doi:10.1016/0022-3093(94)00699-7.
Tiwari R, Mehta N, Shukla R, Kumar A. Kinetic parameters of glass transition in glassy Se1−x Sb x Alloys. Turkish J Phys. 2005;29:233–42.
Patel AT, Pratap A. Study of kinetics of glass transition of metallic glasses. J Therm Anal Calorim. 2012;110(2):567–71. doi:10.1007/s10973-012-2527-8.
Hancock B, Zografi G. The relationship between the glass transition temperature and the water content of amorphous pharmaceutical solids. Pharm Res. 1994;11(4):471–7. doi:10.1023/A:1018941810744.
Mehta N, Kumar A. Comparative analysis of calorimetric studies in Se90M10 (M = In, Te, Sb) chalcogenide glasses. J Therm Anal Calorim. 2007;87(2):345–50. doi:10.1007/s10973-005-7411-3.
Tripathi SK, Patial BS, Thakur N. Glass transition and crystallization study of chalcogenide Se70Te15In15 glass. J Therm Anal Calorim. 2011;107(1):31–8. doi:10.1007/s10973-011-1724-1.
Naqvi SF, Saxena NS. Kinetics of phase transition and thermal stability in Se80−x Te20Zn x (x = 2, 4, 6, 8, and 10) glasses. J Therm Anal Calorim. 2011;108(3):1161–9. doi:10.1007/s10973-011-1857-2.
Lasocka M. The effect of scanning rate on glass transition temperature of splat-cooled Te85Ge15. Mater Sci Eng. 1976;23(2–3):173–7. doi:10.1016/0025-5416(76)90189-0.
Moynihan CT, Easteal AJ, Wilder J, Tucker J. Dependence of the glass transition temperature on heating and cooling rate. J Phys Chem. 1974;78(26):2673–7. doi:10.1021/j100619a008.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6. doi:10.1021/ac60131a045.
Heireche MM, Belhadji M, Hakiki NE. Non-isothermal crystallisation kinetics study on Se90−x In10Sb x (x = 0, 1, 2, 4, 5) chalcogenide glasses. J Therm Anal Calorim. 2013. doi:10.1007/s10973-012-2873-6.
He S, Liu Y, Huang B, Li Z, Wu H. Effect of Zr on glass-forming ability and crystallization kinetics of Y56Al24Co20 metallic glass. J Mater Process Technol. 2008;204(1–3):179–83. doi:10.1016/j.jmatprotec.2007.11.030.
Matusita K, Komatsu T, Yokota R. Kinetics of non-isothermal crystallization process and activation energy for crystal growth in amorphous materials. J Mater Sci. 1984;19(1):291–6. doi:10.1007/BF00553020.
Augis JA, Bennett JE. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J Therm Anal Calorim. 1978;13(2):283–92. doi:10.1007/BF01912301.
Abu El-Oyoun M. DSC studies on the transformation kinetics of two separated crystallization peaks of Si12.5Te87.5 chalcogenide glass: an application of the theoretical method developed and isoconversional method. Mater Chem Phys. 2011;131(1–2):495–506. doi:10.1016/j.matchemphys.2011.10.009.
Pratap A, Raval KG, Gupta A, Kulkarni SK. Nucleation and growth of a multicomponent metallic glass. B Mater Sci. 2000;23(3):185–8. doi:10.1007/BF02719907.
Soltan AS. A study of DSC non-isothermal pre-crystallization kinetics of Pb10Se90 glass using isoconversional kinetic analysis. Phys B. 2010;405(3):965–8. doi:10.1016/j.physb.2009.10.032.
Colmenero J, Barandiaran J. Crystallization of Al23Te77 glasses. J Non-Cryst Solids. 1979;30(3):263–71.
Kaur G, Komatsu T. Crystallization behavior of bulk amorphous Se–Sb–In system. J Mater Sci. 2001;36(18):4531–3. doi:10.1023/A:1017951307399.
Tomolya K, Janovszky D, Sveda M, Hegman N, Solyom J, Roosz A. CuZrAl amorphous alloys prepared by casting and milling. J Phys. 2009;144:012032. doi:10.1088/1742-6596/144/1/012032.
Al-Ghamdi AA, Alvi MA, Khan SA. Non-isothermal crystallization kinetic study on Ga15Se85–x Ag x chalcogenide glasses by using differential scanning calorimetry. J Alloy Compd. 2011;509(5):2087–93. doi:10.1016/j.jallcom.2010.10.145.
Ahmad A, Khan SA, Al-Ghamdi AA, Al-Agel FA, Sinha K, Zulfequar M, et al. Kinetics of non-isothermal crystallization of ternary Se80Te20−x Zn x glasses. J Alloy Compd. 2010;497(1–2):215–20. doi:10.1016/j.jallcom.2010.03.015.
Mehta N, Agarwal P, Kumar A. Calorimetric studies of glass forming ability and thermal stability in a-Se80Te19.5M0.5 (M = Ag, Cd, In, Sb) alloys. Eur Phys J. 2005;31(03):153–8. doi:10.1051/epjap:2005048.
Singh AK, Mehta N, Singh K. Effect of indium additive on glass-forming ability and thermal stability of Se–Zn–Te chalcogenide glasses. Philos Mag Lett. 2010;90(3):201–8.
Hrubý A. Evaluation of glass-forming tendency by means of DTA. Czechoslov J Phys B. 1972;22(11):1187–93. doi:10.1007/BF01690134.
Mehta N, Tiwari RS, Kumar A. Glass forming ability and thermal stability of some Se–Sb glassy alloys. Mater Res Bull. 2006;41(9):1664–72. doi:10.1016/j.materresbull.2006.02.024.
Saad M, Poulain M. Glass forming ability criterion. Mater Sci Forum. 1987;19–20:11–8.
Acknowledgements
The authors like to acknowledge the collaboration with De-Wang Li and stimulating discussions with Rong-Hai Wu.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, X.C., Li, H.Y. Kinetics of non-isothermal crystallization in Cu50Zr43Al7 and (Cu50Zr43Al7)95Be5 metallic glasses. J Therm Anal Calorim 115, 1089–1097 (2014). https://doi.org/10.1007/s10973-013-3364-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3364-0