Abstract
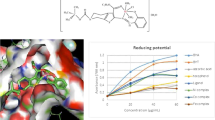
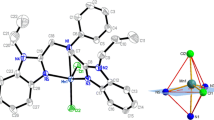
Solid-state M-mef compounds, where M stands for bivalent Mn, Fe, Co, Ni, Cu, or Zn and mef is mefenamate, have been synthesized and characterized by thermoanalytical and spectroscopic techniques, molecular modeling, and antioxidant capacity evaluations. The TG–DTA and DSC curves show that the cobalt, nickel, and zinc compounds were hydrated while the manganese, iron, and copper compounds were anhydrous. The results provided information on the composition, dehydration, thermal stability, ligand denticity, crystallinity, thermal decomposition, as well as the gaseous products that evolved during the thermal decomposition of these compounds. The thermal stability of the anhydrous compounds depended on the nature of the metal ion, and followed the order: Ni > Mn > Zn > Co > Cu–Fe. The antioxidant activity of M-mef was higher than that of mefenamic acid, indicating that they are good models in treating a variety of inflammatory diseases and may lead to the development of new drugs.






Similar content being viewed by others
References
Lozano JJ, Pouplana R, López M, Ruiz J. Conformational analysis of the antiinflammatory fenamates: a molecular mechanics and semiempirical molecular orbital study. J Mol Struct. 1995;335:215–27.
Gilpin RK, Zhou W. Infrared studies of the polymorphic states of the fenamates. J Pharm Biomed Anal. 2005;37:509–15.
SeethaLekshmi S, Guru Row TN. Conformational polymorphism in a non-steroidal anti-inflammatory drug, mefenamic acid. Cryst Growth Des. 2012;12:4283–9.
Kovala-Demertzi D, Staninska M, Garcia-Santos I, Castineiras A, Demertzis MA. Synthesis, crystal structures and spectroscopy of meclofenamic acid and its metal complexes with manganese(II), copper(II), zinc(II) and cadmium(II). Antiproliferative and superoxide dismutase activity. J Inorg Biochem. 2011;105:1187–95.
Bojarowicz H, Kokot Z, Surdykowski A. Complexes of Fe(III) ions with mefenamic acid. J Pharm Biomed Anal. 1996;15:339–42.
Brzyska W, Ożga W. Thermal decomposition of yttrium and lanthanide complexes with mephenamic acid. Thermochim Acta. 1992;195:149–55.
Dimiza F, Papadopoulos AN, Tangoulis V, Psycharis V, Raptopoulou CP, Kessissoglou DP, Psomas G. Biological evaluation of non-steroidal anti-inflammatory drugs–cobalt(II) complexes. Dalton Trans. 2010;39:4517–28.
Lyle SJ, Rahman MDM. Complexometric titration of yttrium and the lanthanons-II. Methods for their determination in oxalates. Talanta. 1963;10:1183–7.
Oliveira CN, Ionashiro M, Graner CA. Titulação Complexométrica de Zinco, Cobre e Cobalto. Ecl Quím. 1985;10:7–10.
Gomes DJC, Caires FJ, Lima LS, Gigante AC, Ionashiro MJ. Synthesis, characterization and thermal study of solid mandelate of some bivalent transition metal ions in CO2 and N2 atmospheres. J Therm Anal Calorim. 2013. doi:10.1007/s10973-011-2189-y.
Becke ADJ. Density-functional thermochemistry. III. The role of exact exchange. Chem Phys. 1993;98:5648–52.
Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev. 1988;37:785–9.
McLean AD, Chandler GS. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J Chem Phys. 1980;72:5639–48.
Krishnan R, Binkley JS, Seeger R, Pople JA. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys. 1980;72:650–4.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, Revision A.02. Gaussian, Inc.: Wallingford, CT; 2009.
Goodson DZ, Sarpal SK. Influence on isotope effect calculations of the method of obtaining force constants from vibrational data. J Chem Phys. 1982;86:659–63.
Dennington R, Keith T, Millam J. GaussView, Version 5.0.8, Semichem., Inc., Shawnee Mission KS, 2000–2008.
Arnao MB. Some methodological problems in the determination of antioxidant activity using chromogen radicals: a practical case. Trends Food Sci Technol. 2000;11:419–21.
De Carvalho CT, Siqueira AB, Ionashiro EY, Pivatto M, Ionashiro M. Synthesis and characterization of solid 2-methoxycinnamylidenepyruvic acid. Ecl Quím. 2008;33:61–8.
Cesur S, Gokbel S. Crystallization of mefenamic acid and polymorphs. Cryst Res Technol. 2008;43:720–8.
Topacli A, Ide S. Molecular structures of metal complexes with mefenamic acid. J Pharm Biomed Anal. 1999;21:975–82.
Caires FJ, Lima LS, Carvalho CT, Siqueira AB, Treu-Filho O, Ionashiro M. Thermal and spectroscopic data to investigate the oxamic acid, sodium oxamate and its compounds with some bivalent transition metal ions. J Therm Anal Calorim. 2011;107:335–44.
British Pharmacopoeia 2009, vol I and II. London: The Stationary Office. 2009.
Acknowledgements
The authors thank Laboratório de Análise Térmica Ivo Giolito (LATIG) IQ-UNESP, computational facilities of Professor Sebastião Claudino da Silva-UFMT, FAPEMAT, CNPq and the CAPES Foundations for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Campos, F.X., Soares, M.R.S., Terezo, A.J. et al. Synthesis, characterization, and antioxidant evaluation of solid-state mefenamates of some bivalent metals. J Therm Anal Calorim 115, 167–176 (2014). https://doi.org/10.1007/s10973-013-3275-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3275-0