Abstract
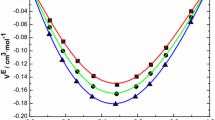
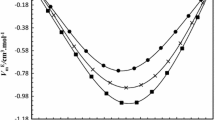
The molar excess enthalpies of eight systems of butylamines + propanols were determined at 298.15 K using a twin-microcalorimeter. All excess enthalpies were exothermic and large. An equilibrium constant K 1 expressed in terms of mole fractions and standard thermodynamic properties of formation (Δf H, Δf G, Δf S) of 1:1 complex were evaluated by ideal mixtures of monomeric molecules and their associated complexes. Concentration dependence of the FT-Raman spectrum showed systematic changes of bands. Spectroscopic considerations based on this and ab initio calculations on molecules were performed at the Mp2/6-311G(d,p) level of theory. Interaction energies between butylamine and propanol were calculated by the supermolecular and NBO methods. The results were discussed with previous results to clarify the steric and positional effect of the amino and hydroxyl group.







Similar content being viewed by others
References
Takeda K, Yamamuro O, Suga H. Thermal study of glass transitions in binary systems of simple hydrocarbons. J Therm Anal. 1992;38:1847–60.
Kimura T, to be published.
Charnd A, Fenby DV. Thermodynamic properties of alcohol-amine mixtures: excess enthalpies of methanol-triethylamine and ethanol-triethylamine. J Chem Eng Data. 1977;22:289–90.
Bender M, Hauser J, Heintz A. Thermodynamics of the ternary mixture (propan-1-ol + triethylamine + n-heptane). Experimental results and ERAS model calculations. Ber Bunsenges Phys Chem. 1991;95:801–11.
Letcher TM. Thermodynamics of aliphatic amine mixtures. I. The excess volumes of mixing for primary, secondary, and tertiary aliphatic amines with benzene and substituted benzene compounds. J Chem Thermodyn. 1972;4:159–73.
Letcher TM, Bayles JW. Thermodynamics of some binary liquid mixtures containing aliphatic amines. J Chem Eng Data. 1971;16:266–71.
Handa YP, Fenby DV, Jones DE. Vapour pressures of triethylamine + chloroform and of triethylamine + dichloromethane. J Chem Thermodyn. 1975;7:337–43.
Domańska U, Marciniak M. Experimental solid–liquid equilibria for systems containing alkan-1-ol + 1,3-diaminopropane: heat capacities of alkan-1-ols and amines-Thermodynamic functions of dissociation and enthalpies of melting of the congruently melting compounds for the systems (alkan-1-ol + amine). Fluid Phase Equilib. 2005;235:30–41.
Reimann RD, Heintz AD. Thermodynamic excess properties of alkanol + amine mixtures and application of the ERAS model. J Solut Chem. 1991;20:29–37.
Funke H, Wetzel M, Heintz A. New applications of the ERAS model. Thermodynamics of amine + alkane and alcohol + amine mixtures. Pure Appl Chem. 1989;61:1429–39.
González JA, Fuente IG, Cobos JC. Thermodynamics of mixtures with strongly negative deviations from Raoult’s law. Part 3. Application of the DISQUAC model to mixtures of triethylamine with alkanols. Comparison with Dortmund UNIFAC and ERAS results. Can J Chem. 2000;78:1272–84.
Saleh MA, Akhtar S, Ahmed MS. Excess molar volumes of aqueous systems of some diamines. J Mol Liq. 2005;116:147–56.
Kimura T, Ozaki T, Takeda S, Nakai Y, Takagi S. Excess enthalpies of binary mixtures of propanediamine + propanediol at 298.15 K. J Therm Anal. 1998;54:285–96.
Kimura T, Kitai T, Kamiyama T, Fujisawa M. Excess enthalpies of binary mixtures of some propylamines + some propanols at 298.15 K. Thermochim Acta. 2006;450:91–5.
Kimura T, Matsushita T, Ueda K, Tamura T, Takagi S. Deutrium isotope effect on excess enthalpies of methanol or ethanol and their deuterium derivatives. J Therm Anal Calorim. 2001;64:231–41.
Kimura T, Usui Y, Nishimura S, Takagi S. Measurement of excess volume of (benzene + cyclohexane) at 298.15 K as a reliability test for a vibration-tube density meter DMA 55. J Fac Sci Technol Kinki Univ. 1989;25:109–16.
Chałasiński G, Szcześniak MM. On the connection between the supermolecular Møller–Plesset treatment of the interaction energy. Mol Phys. 1988;63:205–24.
Gaussian 09, Revision A. Version 7.0, Gaussian, Inc., Pittsburgh PA 2009.
Original data of excess enthalpies of mixtures appear in the online version as supplementary data file.
McGlashan ML, Rastogi RP. The thermodynamics of associated mixtures. Part 1. Dioxan + chloroform. Trans Faraday Soc. 1958;54:496–501.
Fenby DV, Hepler LG. Thermodynamic study of complex formation between dimethyl sulphoxide and chloroform. J Chem Thermodyn. 1974;6:185–9.
Kimura T, Chanoki T, Mizuno H, Takagi S. Excess enthalpies for (carbon tetrachloride+, chloroform+, dimethylchloride + methyl methylthiomethyl sulfoxide) at 298.15 K. Nippon Kagaku Kaishi. 1986;1986:509–13.
Halkier A, Klopper W, Helgaker T, Jørgensen P, Taylor PR. Basis set convergence of the interaction energy of hydrogen-bonded complexes. J. Chem. Phys. 1999;111:9157–67.
Simon S, Duran M, Dannenberg JJ. How does basis set superposition error change the potential surfaces for hydrogen-bonded dimers? J Chem Phys. 1996;105:11024–32.
Cappelli C, Corni S, Mennucci B, Cammi R, Tomasi J. J Phys Chem A. 2002;106:12331–43.
Gausview 05 A. Version 7.0, Gaussian, Inc., Pittsburgh PA 2009.
Hernandez V, López J, Navarrete T. Ab initio study of torsional potentials in 2,2′-bithiophene and 3,4′- and 3,3′-dimethyl-2,2′-bithiophene as models of the backbone flexibility in polythiophene and poly(3-methylthiophene). J Chem Phys. 1994;101:1369–78.
Wang CH, Storms RD. Temperature-dependent Raman study and molecular motion in l-alanine single crystal. J Chem Phys. 1971;55:3291–300.
Shinoda K. Principles of solution and solubilities. New York: Marcel Dekker; 1978.
Israeiachvili JN. Inter-molecular surface force. 2nd ed. San Diego: Academic press; 1992. p. 62.
Keesom PH, Pearlman A. An anomaly in the low temperature atomic heat of silver. Phys Rev. 1952;88:140–1.
Foster JP, Weinhold F. Natural hybrid orbitals. J Am Chem Soc. 1980;102:7211–8.
Reed AE, Curtiss LA, Weinhold F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev. 1988;88:899–926.
Riddick JA, Bunger WB, Sakano TK. Organic solvents. 3rd ed. New York: Wiley; 1986.
Wadsö I. Heat of vaporization for a number of organic compounds at 25 °C. Acta Chem Scand. 1966;20:544–52.
Wadsö I. Enthalpies of vaporization of organic compounds. Acta Chem Scand. 1969;23:2061–4.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kimura, T., Suzuki, T., Takata, K. et al. Excess enthalpies of binary mixtures of butylamines + propanols at 298.15 K. J Therm Anal Calorim 113, 1467–1474 (2013). https://doi.org/10.1007/s10973-013-3226-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3226-9