Abstract
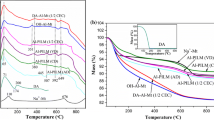
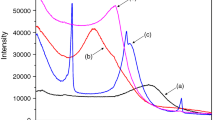
For combining the properties of organoclays and pillared clays, inorganic–organic clays have attracted much attention in recent years. In this study, Al Keggin cation pillared montmorillonites (Al-Mts) were first prepared and parts of Al-Mts were calcined at different temperatures (C-Al-Mts). The inorganic–organic montmorillonites were synthesized by intercalating Al-Mts and C-Al-Mts with the cationic surfactant, hexadecyltrimethyl ammonium bromide (HDTMAB). The products were characterized by X-ray diffraction, X-ray fluorescence, and simultaneous thermogravimetric analysis. For HDTMAB-modified uncalcined Al Keggin cation pillared montmorillonites (H-Al-Mts), the basal spacing increased with the increment of surfactant loading level, but the Al content of H-Al-Mts decreased simultaneously, indicating that the intercalated surfactant replaced some Al Keggin cations in the interlayer space. However, in the case of C-Al-Mts, the interlayer spaces could not be further expanded after surfactant modification, implying that the neighboring montmorillonite layers were “locked” by the aluminum pillars which were formed by dehydroxylation of Al Keggin cation pillars during thermal treatment. The thermal stability of HDTMAB-modified C-Al-Mts (H-C-Al-Mts) was much better than that of H-Al-Mts. The major mass loss of H-C-Al-Mts occurred at ca. 410 °C, corresponding to decomposition of intercalated surfactant cations. In contrast, H-Al-Mts displayed two mass loss temperatures at ca. 270 and 410 °C, corresponding to the evaporation of surfactant molecules and the decomposition of surfactant cations in the interlayer space, respectively.



Similar content being viewed by others
References
Pinnavaia TJ. Intercalated clay catalysts. Science. 1983;220:365–71.
Lahav N, Shani U, Shabtai J. Cross-linked smectites. 1. Synthesis and properties of hydroxy-aluminum-montmorillonite. Clays Clay Miner. 1978;26:107–15.
Lagaly G. Characterization of clays by organic-compounds. Clay Miner. 1981;16:1–21.
He HP, Ma YH, Zhu JX, Yuan P, Qing YH. Organoclays prepared from montmorillonites with different cation exchange capacity and surfactant configuration. Appl Clay Sci. 2010;48:67–72.
Kloprogge JT. Synthesis of smectites and porous pillared clay catalysts: a review. J Porous Mater. 1998;5:5–41.
Occelli ML, Bertrand JA, Gould SAC, Dominguez JM. Physicochemical characterization of a Texas montmorillonite pillared with polyoxocations of aluminum. Part I: the microporous structure. Microporous Mesoporous Mater. 2000;34:195–206.
Ding Z, Kloprogge JT, Frost RL, Lu GQ, Zhu HY. Porous clays and pillared clays-based catalysts. Part 2: a review of the catalytic and molecular sieve applications. J Porous Mater. 2001;8:273–93.
Yuan P, He HP, Bergaya F, Wu DQ, Zhou Q, Zhu JX. Synthesis and characterization of delaminated iron-pillared clay with meso-microporous structure. Microporous Mesoporous Mater. 2006;88:8–15.
Qin ZH, Yuan P, Zhu JX, He HP, Liu D, Yang SQ. Influences of thermal pretreatment temperature and solvent on the organosilane modification of Al13-intercalated/Al-pillared montmorillonite. Appl Clay Sci. 2010;50:546–53.
Kasama T, Watanabe Y, Yamada H, Murakami T. Sorption of phosphates on Al-pillared smectites and mica at acidic to neutral pH. Appl Clay Sci. 2004;25:167–77.
Zhu RL, Zhu LZ, Zhu JX, Ge F, Wang T. Sorption of naphthalene and phosphate to the CTMAB-Al13 intercalated bentonites. J Hazard Mater. 2009;168:1590–4.
Lenoble V, Bouras O, Deluchat V, Serpaud B, Bollinger JC. Arsenic adsorption onto pillared clays and iron oxides. J Colloid Interface Sci. 2002;255:52–8.
Bhattacharyya KG, Gupta SS. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv Colloid Interface Sci. 2008;140:114–31.
Karamanis D, Assimakopoulos PA. Efficiency of aluminum-pillared montmorillonite on the removal of cesium and copper from aqueous solutions. Water Res. 2007;41:1897–906.
Gyftopoulou ME, Millan M, Bridgwater AV, Dugwell D, Kandiyoti R, Hriljac JA. Pillared clays as catalysts for hydrocracking of heavy liquid fuels. Appl Catal A Gen. 2005;282:205–14.
Martinez-Ortiz MJ, Fetter G, Dominguez JM, Melo-Banda JA, Ramos-Gomez R. Catalytic hydrotreating of heavy vacuum gas oil on Al- and Ti-pillared clays prepared by conventional and microwave irradiation methods. Microporous Mesoporous Mater. 2003;58:73–80.
Zhou Q, Frost RL, He HP, Xi YF, Zbik M. TEM, XRD, and thermal stability of adsorbed paranitrophenol on DDOAB organoclay. J Colloid Interface Sci. 2007;311:24–37.
Zhu LZ, Chen BL. Sorption behavior of p-nitrophenol on the interface between anion-cation organobentonite and water. Environ Sci Technol. 2000;34:2997–3002.
Yilmaz N, Yapar S. Adsorption properties of tetradecyl- and hexadecyl trimethylammonium bentonites. Appl Clay Sci. 2004;27:223–8.
Park Y, Frost RL, Ayoko GA, Morgan DL. Adsorption of p-nitrophenol on organoclays. J Therm Anal Calorim. 2013;111:41–7.
Okamoto K, Ray SS, Okamoto M. New poly(butylene succinate)/layered silicate nanocomposites. II. Effect of organically modified layered silicates on structure, properties, melt rheology, and biodegradability. J Polym Sci B Polym Phys. 2003;41:3160–72.
Okada A, Usuki A. Twenty years of polymer-clay nanocomposites. Macromol Mater Eng. 2006;291:1449–76.
Gao Z, Xie W, Hwu JM, Wells L, Pan WP. The characterization of organic modified montmorillonite and its filled PMMA nanocomposite. J Therm Anal Calorim. 2001;64:467–75.
Zhu RL, Wang T, Ge F, Chen WX, You ZM. Intercalation of both CTMAB and Al13 into montmorillonite. J Colloid Interface Sci. 2009;335:77–83.
Ouellet-Plamondon C, Lynch RJ, Al-Tabbaa A. Comparison between granular pillared, organo- and inorgano–organo-bentonites for hydrocarbon and metal ion adsorption. Appl Clay Sci. 2012;67–68:91–8.
Zhu LZ, Zhu RL. Simultaneous sorption of organic compounds and phosphate to inorganic–organic bentonites from water. Sep Purif Technol. 2007;54:71–6.
Li SZ, Wu PX. Characterization of sodium dodecyl sulfate modified iron pillared montmorillonite and its application for the removal of aqueous Cu(II) and Co(II). J Hazard Mater. 2010;173:62–70.
Zhu LZ, Tian SL, Zhu JX, Shi Y. Silylated pillared clay (SPILC): a novel bentonite-based inorgano–organo composite sorbent synthesized by integration of pillaring and silylation. J Colloid Interface Sci. 2007;315:191–9.
An TC, Chen JX, Li GY, Ding XJ, Sheng GY, Fu JM, et al. Characterization and the photocatalytic activity of TiO2 immobilized hydrophobic montmorillonite photocatalysts degradation of decabromodiphenyl ether (BDE 209). Catal Today. 2008;139:69–76.
Occelli ML, Auroux A, Ray GJ. Physicochemical characterization of a Texas montmorillonite pillared with polyoxocations of aluminum. II. NMR and microcalorimetry results. Microporous Mesoporous Mater. 2000;39:43–56.
Thomas SM, Occelli ML. Effects of synthesis conditions on the thermal stability of a Texas montmorillonite expanded with [Al13O4(OH)24(H2O)12]7+ cations. Clays Clay Miner. 2000;48:304–8.
Khalaf H, Bouras O, Perrichon V. Synthesis and characterization of Al-pillared and cationic surfactant modified Al-pillared Algerian bentonite. Microporous Mater. 1997;8:141–50.
Zhu LZ, Zhu RL, Xu LH, Ruan XX. Influence of clay charge densities and surfactant loading amount on the microstructure of CTMA-montmorillonite hybrids. Colloids Surf A. 2007;304:41–8.
Kloprogge JT, Geus JW, Jansen JBH, Seykens D. Thermal-stability of basic aluminum sulfate. Thermochim Acta. 1992;209:265–76.
Pusch R, Yong RN. Microstructure of smectite clays and engineering performance. 1st ed. London: Taylor & Francis; 2006.
Bergaya F, Aouad A, Mandalia T. Pillared clays and clay minerals. In: Bergaya F, Theng BKG, Lagaly G, editors. Handbook of clay science. Amsterdam: Elsevier Science; 2006. p. 393–421.
Acemana S, Lahav N, Yariv S. A thermo-FTIR-spectroscopy analysis of Al-pillared smectites differing in source of charge, in KBr disks. Thermochim Acta. 1999;340–341:349–66.
Zhu JX, Shen W, Ma YH, Ma LY, Zhou Q, Yuan P, et al. The influence of alkyl chain length on surfactant distribution within organo-montmorillonites and their thermal stability. J Therm Anal Calorim. 2012;109:301–9.
He HP, Ding Z, Zhu JX, Yuan P, Xi YF, Yang D, et al. Thermal characterization of surfactant-modified montmorillonites. Clays Clay Miner. 2005;53:287–93.
Acknowledgements
We gratefully acknowledge financial support from the Knowledge Innovation Program of the Chinese Academy of Sciences (Grant No. KZCX2-EW-QN101), the “Strategic Priority Research Program” of the Chinese Academy of Sciences (Grant No. XDB05050200), and the National Natural Science Foundation of China (Grant No. U0933003, 41272060). This is contribution No. IS-1657 from GIGCAS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, L., Zhou, Q., Li, T. et al. Investigation of structure and thermal stability of surfactant-modified Al-pillared montmorillonite. J Therm Anal Calorim 115, 219–225 (2014). https://doi.org/10.1007/s10973-013-3190-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3190-4