Abstract
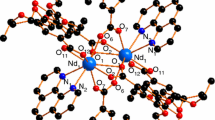
Cerium, praseodymium, and neodymium nitrate complexes with hydrogen bonded hexamethylenetetramine (HMTA) of the formula [Ce(NO3)2(H2O)5](HMTA)2(NO3)(H2O)3, [Pr(NO3)2(H2O)6]2[Pr(H2O)9](HMTA)6(NO3)6(H2O)4 and [Nd(NO3)2(H2O)5](HMTA)2(NO3)(H2O)3 have been prepared and characterized by X-ray crystallography. All the complexes belong to monoclinic crystal system. Ce and Nd complexes have P21/n space group, whereas Pr complex has C2/c. Thermal analyses of these complexes were carried out using TG, DSC, which showed their multi-step decomposition. Kinetics of thermolysis has been done by applying model fitting as well as model free isoconversional method. In order to see the response of rapid heating, ignition delay measurements were carried out. The thermal decomposition pathways have also been demonstrated. On the basis of thermal studies the thermal stability of the complexes was found in the order; Pr > Ce > Nd. In order to identify the end products of thermolyses, X-ray diffraction patterns of end product were carried out which showed the formation of corresponding metal oxides.













Similar content being viewed by others
References
Carmona D, Lamata MP, Oro LA. Recent advances in homogeneous enantioselective Diels–Alder reactions catalysed by chiral transition–metal complexes. Coord Chem Rev. 2000;200–202:717–72.
Epstein DM, Chapell LL, Khalili H, Supkowski RM, Horrocks WD, Morrow JR Jr. Eu(III) macrocyclic complexes promote cleavage of and bind to models for the 5′-cap of mRNA. Effect of pendent group and a second metal ion. Inorg Chem. 2000;39(10):2130–4.
Feng J, Sun G, Pei F, Liu M. Comparison between GdDTPA and two gadolinium polyoxometalates as potential MRI contrast agents. J Inorg Biochem. 2002;92:193–9.
Sessler JL, Miller RA. New drugs with diverse clinical applications in radiation and photodynamic therapy. Biochem Pharmacol. 2000;59:733–9.
Ulusoy U, Whitley JE. Determination of intestinal uptake of iron and zinc using stable isotopic tracers and rare earth markers. Nutr Res. 1999;19(5):675–88.
Agwara MO, Ndifon PT, Ndikontar MK. Physicochemical studies of some hexamethylenetetramine metal(II) complexes. Bull Chem Soc Ethiopia. 2004;18:143–8.
Xuan YW, Wu W, Li SJ. Synthesis and crystallographic characterization of a six coordinate Cu(II) complex based on hexamethylenetetramine ligand. Cryst Res Technol. 2009;44:127–30.
Li F, Ma J, Song S, Yang J, Liu Y, Su Z. Influence of neutral ligands on the structures of silver(I) sulfonates. Inorg Chem. 2005;44:9374–83.
Zheng Y, Ying E. Malonato-bridged hexamethylenetetramine coordination polymers containing Mn(II) and Cu(II). J Coord Chem. 2005;58(5):453–60.
Lim MJ, Murray CA, Tronic TA, deKrafft KE, Ley AN, deButts JC, Pike RD, Lu H, Patterson HH. Copper(I) cyanide networks: synthesis, structure, and luminescence behaviour. Part 2. Piperazine ligands and hexamethylenetetramine. Inorg Chem. 2007;47:6931–47.
Larionov SV, Kokina TE, Glinskaya LA, Klevtsova RF. Nickel(II) diisobutyldithiophosphinate complexes with hexamethylenetetramine and triethylenediamine: synthesis and properties. Crystal and molecular structure of [Ni2(C6H12N4){(i-C4H9)2PS2}4]. Russ J Coord Chem. 2002;28(8):560–4.
Zhang Y, Li J, Nishiura M, Imamoto T. Structural, spectral and thermal properties of a new anion zinc(II) hexamethylenetetramine complex. J Mol Struct. 2002;523:257–60.
Liittringhauusn A, Kullick W. Gemiscrhte carbonyl komplexe des Cr0 und des Mo0 mit organo-stickstoffverbindungen. Tetrahedron Lett. 1959;10:13–5.
Zheng S, Tong M, Chen X. Silver (I)–hexamethylenetetramine molecular architectures: from self-assembly to designed assembly. Coord Chem Rev. 2003;246:185–202.
Xue H, Gao H, Twamley B, Shreeve JM. Energetic nitrate, perchlorate, azide and azolate salts of hexamethylenetetramine. Eur J Inorg Chem. 2006;15:2959–65.
Cheng C, Gong S, Fu Q, Shen L, Liu Z, Qiao Y, Fu C. Hexamethylenetetramine as both a ligand and a reducing agent in AGET atom transfer radical batch emulsion polymerization. Polym Bull. 2011;66:735–46.
Sieranski T, Kruszynski R. Magnesium sulphate complexes with hexamethylenetetramine and 1,10-phenanthroline—thermal, structural and spectroscopic properties. J Therm Anal Calorim. 2012;109:141–52.
Konar S, Mukherjee PS, Drew MGB, Ribas J, Chaudhari NR. Synthesis of two new 1D and 3D networks of Cu(II) and Co(II) using malonate and urotropine as bridging ligands: crystal structures and magnetic studies. Inorg Chem. 2003;42:2545–52.
Dalvi AA, Satpati AK, Palrecha MM. Simultaneous determination of Pt and Rh by catalytic adsorptive stripping voltammetry, using hexamethylenetetramine (HMTA) as complexing agent. Talanta. 2008;75:1382–7.
Fedoroff BT, Sheffield OE. Encyclopedia of explosives and related items, vol. 5. Dover: Picatinny Arsenal; 1966. p. E95.
Koper JH, Jansen OG, van den Berg PJ. Delft Technische Hogeschool. Netherlands: Explosivstoffe; 1970. p. 181–3.
Singh G, Kapoor IPS, Pandey DK. Hexammine metal perchlorates as energetic burning rate modifiers. J Energ Mater. 2002;20:223–44.
Singh G, Pandey DK. Studies on energetic compounds, part 27: kinetics and mechanism of thermolysis of bis(ethylenediamine) metal nitrates and their role in the burning rate of solid propellants. Propellants Explos Pyrotech. 2003;28(5):231–9.
Singh G, Barnawal BP, Kapoor IPS, Kumar D, Singh CP, Frohlich R. Preparation, X-ray crystallography and thermal decomposition of some transition metal nitrate complexes with hexamethylenetetramine. J Therm Anal Calorim. 2008;91(3):971–7.
Singh G, Barnawal BP, Kapoor IPS, Kumar D, Frohlich R. Preparation, X-ray crystallography and thermal decomposition of some transition metal perchlorate complexes with hexamethylenetetramine. J Phys Chem A. 2007;111:12972–6.
Singh G, Shrimal AK, Kapoor IPS, Singh CP, Kumar D, Mannan SM. Kinetics of thermolysis of some transition metal perchlorate complexes with 1,6-diaminohexane ligand. J Therm Anal Calorim. 2011;103:149–55.
Singh G, Singh CP, Fröhlich R. Preparation, characterization and thermolysis of metal nitrate complexes with 4,4′-bipyridine. J Therm Anal Calorim. 2006;85:425–31.
Kumar D, Kapoor IPS, Singh G, Goel N, Singh UP. Preparation, X-ray crystallography and thermolysis of transition metal nitrates of 2,2′-bipyridine. J Therm Anal Calorim. 2012;107:325–34.
Kumar D, Kapoor IPS, Frohlich R, Singh G. Preparation, characterization, and kinetics of thermolysis of nickel and copper nitrate complexes with 2,2′-bipyridine ligand. Thermochim Acta. 2012;545:67–74.
Trzesowsk-Kruszynska A, Kruszynski R, Zalewicz M, Bartczak TJ. Coordination sphere geometry changes of lanthanoid(III)nitrate complexes with hexamethylenetetramine. J Coord Chem. 2010;63(6):1013–28.
Sheldrick GM. SADABS, Program for scaling and correction of area detector data. Göttingen: University of Göttingen; 1996.
Sheldrick GM. Phase annealing in SHELX-90: direct method for larger structures. Acta Crystallogr A. 1990;46:467–73.
Sheldrick GM. SHELXTL-NT, version 6.12. Reference manual. Göttingen: University of Göttingen; 2000.
Klaus B. DIAMOND, version 1.2c. Bonn: University of Bonn; 1999.
Allen FH. The Cambridge structural database: a quarter of a million crystal structures and rising. Acta Crystallogr B. 2002;58:380–8.
Singh G, Singh RR. Indigenously fabricated apparatus for thermogravimetric analysis. Res Ind. 1978;23:92–3.
Brown ME, Dollimore D, Galway AK. Reactions in the solid state, comprehensive chemical kinetics, vol. 22. Amsterdam: Elsevier; 1977. p. 1–340.
Vyazovkin S, Wight CA. Isothermal and nonisothermal reaction kinetics in solids: in search of ways toward consensus. J Phys Chem A. 1997;10:8279–84.
Singh G, Kapoor IPS, Vasudeva SK. Thermolysis of AP-PS-additive mixtures. Indian J Technol. 1991;29:584–9.
Freeman ES, Gorden S. The application of the absolute rate theory of the ignition of propagatively reacting systems: thermal ignition of the system, lithium nitrate–magnesium, sodium nitrate–magnesium. J Phys Chem. 1956;60:867–71.
Zinn J, Rogers RN. Thermal initiation of explosives. J Phys Chem. 1962;66:2646–53.
Acknowledgements
Thanks are due to Head, Chemistry Department, DDU Gorakhpur University, Gorakhpur for lab facilities. The financial assistance UGC for Emeritus Fellow to Dr. Gurdip Singh and CSIR for SRF to Dinesh Kumar is also acknowledged. Authors are also thankful to Institute Instrumentation Centre, IITR, Roorkee for TG-DSC and Chairman, Department of Materials Engineering, IISc, Bangalore for providing XRD facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, D., Kapoor, I.P.S., Singh, G. et al. Lanthanoid metal nitrates with hydrogen bonded hexamethylenetetramine. J Therm Anal Calorim 114, 5–18 (2013). https://doi.org/10.1007/s10973-012-2826-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2826-0