Abstract
The thermal properties of new anion exchangers consisting of silica core and organic layers grafted onto their surface were evaluated by thermogravimetry and differential scanning calorimetry. The anion exchangers were prepared by chemical modification of bare silica gel. The support was coated with polymeric layers formed by condensation polymerization of primary amines with diepoxides. Synthesized copolymers of methylamine and 1,4-butanedioldiglycidyl ether have a dendrimer structure. By TG/FTIR/MS, it was observed that silica-layered materials exhibited two stages of mass loss: first with T max1 160 °C, connected with desorption of water molecules from the adsorbents surface, and then with T max2 390 °C, connected with degradation of organic layers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last few decades, polymeric microspheres with developed porous structure and containing functional groups have attracted a great deal of attention. They are often applied as stationary phases in chromatography, including also ion chromatography (IC) [1–3]. Since IC offers many possibilities of application, in recent years the number of developed ion-exchange materials has increased dynamically [4–7]. Most stationary phases currently used for separation of ionic compounds are based on organic polymers as well as silica gel. In contrast to stationary phases prepared on silica gel, organic polymers show higher stability toward in extreme pH conditions. The silica-based anion exchangers [8, 9] can be operated only in between pH 2.0–9.5 while polymeric ion exchangers are stable across the entire pH range. Thus, styrene/divinylbenzene (PS/DVB) copolymers [10, 11] and polymethacrylate resins [12, 13] are the most important organic polymers used as materials in the manufacturing polymer-based anion exchangers. One of the most popular anion-exchange groups in the structure of the stationary phases used in IC are the quaternary ammonium groups [14, 15]. This type of stationary phases exhibit good selectivity for the separation of inorganic and organic anions. One of the methods which allows to obtain quaternary ammonium groups in the structure of anion exchangers is condensation polymerization of primary amine with diepoxide [methylamine (MA) and 1,4-butanedioldiglycidyl ether (BDDE)] [15–19]. In this type of synthesis, the tertiary amine and quaternary ammonium groups are formed which are anion exchange centers when they are used in ion chromatography.
In this study, a newly functionalized stationary phase containing quaternary ammonium groups was reported. Based on the methodology introduced by Pohl and coworkers [16, 17], the hyperbranched anion-exchange polymer stationary phase was prepared. Novel anion exchangers with dendrimer structure were prepared by chemical modification of silica surface with methyl amine and BDDE. This approach introduced covalent bonds between the silica surface and the organic layers. Controlled synthesis allows for the formation of a defined number of bonded layers.
This paper presents a study of the thermal properties of silica-supported organic materials with dendrimer structure using differential scanning calorimetry (DSC) and thermogravimetry coupled with FTIR and MS to analyze the evolved products during their decomposition. In addition, ATR-FTIR spectroscopic studies of structural changes in the partially degraded materials were performed.
Experimental
Materials
The composite silica-organic materials were prepared by a modification of silica gel Kromasil 100 (Akzo Nobel, Sweden). The following reagents were used for the chemical modification of the silica gel support: [3-(2-aminoethylamino)propyl] trimethoxysilane, MA, and BDDE that were obtained from Sigma-Aldrich Chemie (Steinheim, Germany). The toluene, methanol, and hexane of reagent grade used for purification of obtained composite materials were from POCh (Gliwice, Poland).
Preparation of anion exchangers
Figure 1 shows the chemical structure of the anion-exchanger. This material was prepared by a modification of silica gel Kromasil 100 with a particle diameter of 5 μm. Modification of the silica gel with organic ligands containing amino groups was performed according to methodology described earlier [20]. Silica gel was dried at 180 °C under vacuum to remove adsorbed water. Further, the 3-(2-aminoethyloamino)propylsilane was added. The reaction was carried out for 12 h at the temperature 80 °C. Modified silica gel was flushed with toluene, methanol, and hexane and then dried. The obtained material was used as a support for anion exchanger synthesis during condensation polymerization. At this step of preparation, MA and BDDE were used as monomers to build a branching polymer that possesses terminal quaternary ammonium anion exchanger sites. A 15 mL of BDDE monomer (7.0–7.4 %) was added to the resin and reacted for 30 min at the temperature of 60–70 °C. After the reaction the mixture was filtered and rinsed with redistilled water. Following the addition of 15 mL 3.0–5.0 % (v/v) of MA was added to the resin and reacted for 30 min at the temperature 60–70 °C, filtered and rinsed with redistilled water. These two steps allow to obtain a layer of anion exchanger on the support surface. The process was repeated two, three, four, seven, eleven, and fifteen times to make 2, 3, 4, 7, 11, and 15-layers on the solid support, respectively.
Methods of analysis
Elemental analysis (CHN) was made using a Perkin-Elmer CHN 2450 analyzer (Palo Alto, CA, USA). ATR-FTIR spectra were recorded on a Tensor 27 (Bruker) spectrometer equipped with diamond crystal. The spectra were recorded in the spectral range of 600–4,000 cm−1 with a resolution of 4 cm−1 and 50 scans.
Thermogravimetric analysis of materials was carried out with a Netzsch STA 449 F1 Jupiter thermal analyzer (Germany) at the heating rate of 10 K min−1 in helium, in the temperature range of 20–850 °C and with the sample mass of 10 mg. Helium flow was 20 mL min−1. The gas composition evolved during the decomposition process was detected and analyzed by quadrupole mass spectrometer QMS 403C Aëolos (Germany) as well as FTIR spectrometer Tensor 27 Bruker (Germany) coupling on-line to a STA instrument. The mass spectrometer was connected on-line to a STA instrument by a quartz capillary heated to 300 °C. The QMS was operated with an electron impact ionizer with the energy of 70 eV. The measurements performed in scan mode for m/z, where m is the mass of the molecule and z is the charge of the molecule in electron charge units in the range of 10–100 amu.
The degradation profile was followed not only from the analysis of the evolved volatile products, but also through the changes in the material surface after each weight loss. In this case, the residual masses of materials from TGA were analyzed by ATR-FTIR using an IR spectrometer with the resolution of 4 cm−1 and 50 scans.
Calorimetric measurements were carried out with the Netzsch DSC 204 calorimeter (Germany) operating in the dynamic mode. The dynamic scans were performed at the heating rate of 10 K min−1 from room temperature to the maximum of 550 °C under argon atmosphere (30 mL min−1) in two stages. The first scan was performed from room temperature to the maximum of 120 °C to remove any adsorbed moisture, especially water. The second scan was conducted between 20 and 550 °C. The mass of the sample was 10 mg. As a reference an empty aluminum crucible was used.
Results and discussion
As a result of chemical modification of silica gel with methyl amine and BDDE new types of anion exchangers were obtained (Fig. 1). The support surface was covered with 1, 2, 4, 7, 11, and 15 organic layers possessing in their structure different types of amine groups including quaternary ammonium groups which are the most desirable from the practical point of view. The aim of this study was to obtain the thermal characteristics of the prepared silica-layer materials.
The multi-step grafting method used to immobilize the organic layers onto silica was assessed by elemental analysis and IR measurements. The results from CHN analysis given in Table 1 show that organic layers were successfully grafted onto silica surface. Figure 2 presents exemplary ATR-FTIR spectra of the new materials. In the spectrum of pure silica support, strong absorption peaks at 1,073 and 793 cm−1 attributed to the Si–O–Si stretch are visible [21]. On the spectra of the covered materials new absorption peaks are present, and their intensity increase with the increasing number of organic layers. Covered silica supports give new bands at 1,458 cm−1 which can be attributed to the deformation vibration of the –CH2– group adjacent to N–H groups. Absorption peaks at 1653, 1365 and 1329 cm−1 are attributed to the N–H band of the amine groups. Peaks at 2,960 and 2,860 cm−1 are characteristic to the C–H stretching of methylene groups. The peak at 1,066 cm−1 overlapping with the strong peak of silica support corresponds to the C–N vibration. The peak at 3,360 cm−1 corresponds to the –OH group vibration present in the structure of materials and also in the water absorbed on the surface of the anion exchangers.
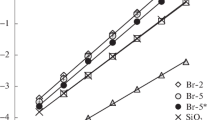
The DSC was performed in an argon atmosphere of 20–550 °C. The DSC curves of all silica-layers materials are presented in Fig. 3. For pure silica, almost any changes on the DSC curve are observed. Grafting on the surface of silica organic layers changes the character of the DSC curves completely. For the covered materials on the DSC curves, two peaks are observed. The first endothermic peaks with the onset at ~130 °C are connected with the desorption of water absorbed on the porous structure of the materials [22–24]. It is clearly visible that along with the increased number of organic layers the heat of thermodesorption increases. The second exothermic peak is observed at the onset of 388–395 °C. It is associated with the decomposition of the organic part of the anion exchangers. The heat of the decomposition reactions increases with the increasing number of organic layers grafted onto the silica surface. Since DSC experiments were conducted in argon atmosphere, it could be expected that decomposition of organic layers should be endothermic processes. However, the presence of oxygen in the structure of the materials initiated autocatalytic exothermic oxidation of organic layers. This observation was also confirmed by thermogravimetric analysis of composite silica-polymeric materials which was performed in helium atmosphere. Their TG weight loss curves and corresponding derivate curves are shown in Figs. 4 and 5, respectively. In addition, the characteristic data obtained from the TG analysis are given in Table 2. By comparing the thermal degradation behavior of the obtained materials, it was observed that their degradation profile changes with the number of the organic layers grafted onto the silica surface. Pure silica had a slight weight loss at 300 °C, the loss caused by the hydroxyl group according to the literature [25]. Since the inorganic part (silica) of the composite materials lost almost none of its weight during the heating period, all of the weight loss of the studied materials should only come from the degradation of the organic part. Comparing data obtained from the TG and results from CHN analysis, it can be seen that with the increase of organic content of materials, the weight loss increases. According to previous studies, addition of silica particles into a polymer did not significantly alter the thermal degradation mechanism of the polymer part of polymer-silica composite materials [26, 27]. The covered materials exhibited two separate degradation steps with two maximum rate peaks: T max1 at 160–163 °C and T max2 at 379–403 °C. The FTIR and MS analysis of the evolved gases produced some information about the products of the degradation of composite materials. Figure 6 shows Gram–Schmidt plots that provide information about the total IR absorbance of the evolved components over the entire spectral range [28]. The first peak on the DTG and Gram–Schmidt plots are connected with the evolving of water from the structure of the prepared materials. The next peaks are connected with the degradation of the organic parts.
The FTIR spectra for the 3, 7, 11, and 15-layers silica materials presented in Fig. 7 correspond to the maximum of the second Gram–Schmidt peaks at about 390 °C. The FTIR spectra reveal fragments representative of aliphatic groups (2944–2827, 1380 cm−1), CO2 (2,354 cm−1) and CO (2,171 and 2,111 cm−1), C–O–C vibration (1,121 cm−1) and C–N vibration (1,055 cm−1). In the carbonyl region, a band at ca. 1,740 cm−1 with a shoulder at ca. 1,650 cm−1 was also noticed and might be related to the evolving of C=O groups presented probably in aldehyde fragments, which are formed during intramolecular oxidation and fragmentation of organic layers. The MS profile of the evolved gases and the total ion current (TIC) is presented in Fig. 8. Evolution of water (m/z = 18), carbon dioxide (m/z = 44), amine fragments (m/z = 28, 29), carbon oxide (m/z = 28), aliphatic fragments (m/z = 15, 42, 43, 56), and aldehyde (m/z = 40, 41) was detected. Moreover, evolution of carbon dioxide was also observed at about 600 °C and was connected with the final decomposition of the materials.
In order to follow the changes in silica-layer materials during the heating at different stages of decomposition, the remaining material was also analyzed by ATR-FTIR and Fig. 9 shows spectra of initial and decomposed 15-layers silica material. Taking into account that the spectra before and after heating to 230 °C (the first step of decomposition) show only small changes in the region of about 3,400 cm−1, we can conclude that the first peak in the DTG and Gram–Schmidt plots is associated with the loss of adsorbed water, not with the structural decomposition of the organic parts of the composite material [29]. The spectrum of the material degraded at 590 °C corresponds to that of pure silica and suggests that the organic part evolved from the material. Hence, we do not treat the first weight loss on the TG curves as a decomposition of dendrimeric material, and the initial decomposition temperature (IDT) was determined as the temperature of 5 % weight loss. Due to the increasing number of organic functional groups on the surface of silica composite materials which interact with water molecules, an increase of weight loss in the range of 1.2–4.8 % connected with water desorption is observed. The determined IDT are in the range of 236–362 °C and decrease along with the increasing number of organic layers. As pure silica lost almost none of its weight during the heating period, the shift of the IDT to a high temperature region might simply be due to the fact that the materials possess a relatively small amount of organic parts. That is to say, the thermal stability of the prepared anion exchangers was not enhanced with the addition of silica particles.
Conclusions
It was concluded that grafted composite silica-organic materials can be obtained by condensation polymerization of primary methylamine amine with BDDE onto the surface of bare silica. The synthesized anion exchangers exhibited good thermal stability, suitable for their use in chromatographic experiments carried out with higher temperatures. DSC and TG measurements indicated that initial decomposition temperatures of the investigated anion exchangers is in the range of 236–362 °C and increases along with the decrease of the number of the organic layers. On the other hand, the silica core of the prepared materials did not alter the thermal degradation behaviors of the organic layers. Degradation starts from 236 to 362 °C and is preceded by desorption of the absorbed water in 160 °C.
References
Small H, Stevens TS, Bauman WS. Novel ion exchange chromatographic method using conductimetric detection. Anal Chem. 1975;47:1801–9.
Saari-Nordhaus R, Anderson JM. Dual-column techniques for the simultaneous analysis of anions and cations. J Chromatogr A. 1992;602:127–33.
Yan D, Schwedt G. Simultaneous ion chromatography of inorganic anions together with some organic anions and alkaline earth metal cations using chelating agents as eluents. J Chromatogr A. 1990;516:383–93.
Li R, Lee WL, Takeuchi T. Determination of iodide in seawater using C30 column modified with polyoxyethylene oleyl ether in ion chromatography. Talanta. 2007;72:1625–9.
Chen ZL, Megharaj M, Naidu R. Speciation of iodate and iodide in seawater by non-suppressed ion chromatography with inductively coupled plasma mass spectrometry. Talanta. 2007;72:1842–6.
Bruzzoniti MC, De Carlo RM, Fungi M. Simultaneous determination of alkali, alkaline earths and ammonium in natural waters by ion chromatography. J Sep Sci. 2008;31:3182–9.
Yoshkawa KJ, Okamura M, Inokuchi M, Akio S. Ion chromatographic determination of organic acids in food samples using a permanent coating graphite carbon column. Talanta. 2007;72:305–9.
Matsushita S, Tada Y, Baba N, Osako K. High-performance ion chromatography of anions. J Chromatogr. 1983;259:459–64.
Vydac HPLC columns and separation materials. Hesperia: The Separation Groups; 1990–1991. p. 20–3.
Weiss J, Jensen D. Modern stationary phases for ion chromatography. Anal Bioanal Chem. 2003;375:81–98.
Gawdzik B, Matynia T, Osypiuk J. Porous copolymer of the methacrylic ester of dihydroxydiphenylmethane diglycidyl ether and divinylbenzene as an HPLC packing. Chromatographia. 1998;47:509–14.
Weiss J. Handbook of ion chromatography. 3rd ed. Weinheim: VCH; 2004.
Podkościelna B. The highly crosslinked dimethacrylic/divinylbenzene copolymers. Characterization and thermal studies. J Therm Anal Calorim. 2011;104:725–30.
Hill DJ, O’Donnell JH, Pomery PJ, Whittaker MR. A high resolution NMR investigation into the microstructure of HEMA and EEMA copolymers. Polymer Gels Netw. 1995;3:85–97.
Creed JT, Magnuson ML, Pfaff JD, Brockhoff C. Determination of bromate in drinking waters by ion chromatography with inductively coupled plasma mass spectrometric detection. J Chromatogr A. 1996;753:261–7.
Kubań P, Dasgupta PK, Pohl C. Open tubular anion exchange chromatography. Controlled layered architecture of stationary phase by successive condensation polymerization. Anal Chem. 2007;79:5462–7.
Pohl C, Saini C. New developments in the preparation of anion exchange media based on hyperbranched condensation polymers. J Chromatogr A. 2008;1213:37–44.
Buszewski B, Jaćkowska M, Bocian S, Kosobucki P, Gawdzik B. Functionalized polymeric stationary phases for ion chromatography. J Sep Sci. 2011;34:601–8.
Smith MB, March J. Advanced organic chemistry: reactions, mechanisms, and structure. 5th ed. New York: Wiley; 2001.
Buszewski B, Jezierska M, Ostrowska-Gumkowska B. Silicon dioxide surface modified with cholesterol derivative. Mater Chem Phys. 2001;72:30–41.
Boven G, Michiel LCMO, Challa G, Arend JS. Grafting kinetics of poly(methyl methacrylate) on microparticulate silica. Polymer. 1990;31:2377–83.
Barczak M, Oszust-Cieniuch M, Borowski P, Fekner Z, Zięba E. SBA-15 silicas containing sucrose. Chemical, structural, and thermal studies. J Therm Anal Calorim. 2012;108:1093–9.
Popescu LM, Piticescu RM, Stoiciu M, Vasile E, Trusca R. Investigation of thermal behaviour of hybrid nanostructures based on Fe2O3 and PAMAM dendrimers. J Therm Anal Calorim. 2012. doi:10.1007/s10973-012-2352-0.
Bolbukh Y, Tertykh V, Klonos P, Pissis P. DSC study of polyhydroxyethylmethacrylate filled with modified silicas. J Therm Anal Calorim. 2012;108:1111–9.
Zhang W, Lee H-R. Grafting of polyethylene glycols onto nanometer silica surface by 1,4-phenylene diisocyanate. Surf Interface Anal. 2010;42:1495–8.
Liu YL, Hsu CY, Wei WL, Jeng RJ. Preparation and thermal properties of epoxy–silica nanocomposites from nanoscale colloidal silica. Polymer. 2003;44:5159–67.
Kashiwagi T, Morgan AB, Antonucci JM, Van Landingham MR, Harris RH, Award WH, et al. Thermal and flammability properties of a silica–poly(methylmethacrylate) nanocomposite. J Appl Polym Sci. 2003;89:2072–8.
Marini A, Berbenni V, Capsoni D, Riccardi R, Zerlia T. Factors affecting the spectral response in a TG/FT-IR experiment. Appl Spectrosc. 1994;48:1468–71.
Dong D, Liu X, Hu W. The structure and mechanism of porous silica films by sol–gel method using poly(ethylene glycol) and side-chain polyether modified polydimethylsiloxane with terminal Si–CH3 as templates. J Mater Sci Mater Electron. 2011;22:944–8.
Acknowledgements
The authors would like to thank Dr Szymon Bocian for his help in the preparation of new anion exchangers. The research was carried out with the equipment purchased; thanks to the financial support of the Operational Programme Development of Eastern Poland 2007–2013.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Grochowicz, M., Gawdzik, B., Jaćkowska, M. et al. Investigation of the thermal behavior of new silica-polymer anion exchangers. J Therm Anal Calorim 112, 885–891 (2013). https://doi.org/10.1007/s10973-012-2635-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2635-5