Abstract
The evaluation of the possibilities to use coal-tar pitch modified with waste poly(ethylene terephthalate) or phenol–formaldehyde resin for the preparation of activated carbons was carried out. The measurement of thermal analysis (DSC), softening point, coking value, content of components insoluble in toluene and quinoline of pitch-polymer compositions were carried out. Coal-tar pitch and pitch-polymer compositions were carbonized and activated with steam at 800 °C to 50 % burn-off. For the obtained activated carbons the determination of thermal analysis (DSC), BET surface area on the basis of volumetric low-temperature adsorption of nitrogen, mesopore, and micropore volume from benzene adsorption/desorption isotherms (gravimetric McBain-Bakr method) were carried out.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Activated carbons (AC) are most often produced from natural feedstocks, such as hard coal, lignite, wood, peat, stones, and peels of the fruits [1–3]. Based on the literature data, a conclusion can be drawn that also synthetic polymers can be used for the preparation of AC [4, 5]. Coal-tar pitch (CTP) modified with polymers, e.g., poly(ethylene terephthalate) (PET), phenolic resins, polyacrylonitrile and polyvinylpyridine, can also be a precursor of carbon adsorbents. [6–8].
In the Institute of Chemistry of Warsaw University of Technology in Plock, studies on modification of bitum materials with polymers in order to improve their usable properties, have been carried out for many years [9–13]. Investigations were also undertaken on the use of CTP and pitch-polymer compositions for the production of porous materials [14–17]. On the basis of obtained results it was found that the raw materials for the production of AC or mineral-carbon sorbents are, i.e., CTPes and pitch-polymer compositions containing, e.g., significant amount of components insoluble in quinoline. One of the factors making possible the evaluation of usefulness of coal derived bitumens modified with polymers for the preparation of AC is the determination of their thermal properties. Thermal properties of CTPes and pitch-polymer compositions are evaluated mainly on the basis of the measurement of softening point. In this study, DSC method was proposed for this evaluation. An attempt was made to determine the influence of PET or phenol-formaldehyde resin (PF) addition on thermal properties of CTP as well as AC obtained from pitch-polymer compositions.
Experimental procedures
The raw materials used in this study were CTP and selected municipal waste polymers: PET and PF. Pitch-polymer compositions containing from 10 to 70 wt% waste were prepared in the conditions allowing to obtain homogeneous and stable mixtures. Depending on the applied municipal waste polymer, the components were homogenized in the temperatures from 150 to 260 °C, during 0.5–2.5 h. The composition of mixtures and preparation conditions are presented in Table 1.
For CTP and pitch-polymer compositions the following measurements were carried out:
-
thermal properties by the means of scanning differential calorimeter Netzsch Maia F3. The measurements were carried out in the range of temperatures from (−100) to 530 °C, with the temperature increase rate of 10 K/min,
-
softening point by “Ring and Ball” method (SP) according to the PN-EN 1427:2001 standard,
-
coking value (CV) according to the PN-C-97093:1993 standard,
-
content of components insoluble in toluene (TI) according to the method elaborated in the Institute of Chemistry, Warsaw University of Technology in Plock,
-
content of components insoluble in quinoline (QI) according to the PN-C-97058:1999 standard.
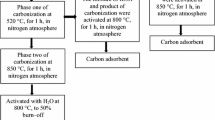
CTP and prepared pitch-polymer compositions were carbonized and activated with steam. Carbonization was carried out in two stages:
-
first stage of initial carbonization was conducted by heating the sample to the temperature of 520 °C with the heating rate of 5 K/min, in nitrogen atmosphere and annealing it in this temperature for 1 h,
-
second stage of carbonization was carried out in a horizontal furnace by heating the sample to the temperature of 520 °C with the heating rate of 15 K/min, and then to 850 °C with the heating rate of 10 K/min. The sample was annealed at 850 °C for 1 h.
Prepared carbonizates were activated with steam at 800 °C to 50 % burn-off. For the samples after the activation process the measurements of thermal analysis (DSC) in the range of temperatures from (−25) to 530 °C, with the temperature increase rate of 10 K/min. In addition, for the obtained AC the determination of BET surface area on the basis of volumetric low-temperature adsorption of nitrogen, mesopore and micropore volume from benzene adsorption/desorption isotherms (gravimetric McBain-Bakr method) were carried out. With the use of “Izotermy” computer software the calculations of mesopore (on the basis of Dollimore–Heal equation) and micropore (on the basis of Dubinin–Radushkevich equation) volume were conducted [18].
Results and discussion
Pitch-PET compositions
In Fig. 1, DSC curves of CTP, PET and selected pitch-polymer compositions are presented. Table 1 contains selected thermal and physicochemical properties of CTP and pitch-polymer compositions.
Temperatures and enthalpies (ΔH) of phase transitions of the CTP attained the following values: the transition from the brittle to the viscoelastic state (51.2 °C, ΔH = −2.386 J/g), melting point (97.4 °C, ΔH = −0.030 J/g), onset of decomposition (307.6 °C).
Based on the course of DSC curves of pitch-PET compositions (Fig. 1) it was found that transitions characteristic for both CTP and waste PET occurred in them as an effect of temperature, including:
-
endothermic transition, occurring for respective compositions in temperatures ranging from 56.6 to 74.7 °C (enthalpy values from −0.380 to −3.221 J/g), related probably to the transition of pitch from brittle to viscoelastic state,
-
glass transition of PET, occurring for respective compositions in the range of temperatures from 67.3 to 88.9 °C (heat capacity (ΔC p ) values from 0.017 to 0.146 J/(g K)),
-
endothermic transition, occurring in the temperatures of ca. 103 °C (enthalpy values from −0.013 to −0.230 J/g), related probably to pitch melting,
-
crystallization of PET, occurring for respective compositions in the range of temperatures from 154.9 to 188.7 °C (enthalpy values from 0.815 to 26.250 J/g) [19],
-
endothermic transition, occurring for respective compositions in the range of temperatures from 213.6 to 233.9 °C (enthalpy values from −0.673 to −35.850 J/g), related probably to PET melting,
-
endothermic transition, related probably to decomposition of samples. The onset of this transition for studied compositions occurred in the temperatures from 313.0 to 397.1 °C.
On the DSC curve for the composition containing 50 wt% of PET (Fig. 1a), an exothermic transition occurring at 140.9 °C was observed. It indicates probably the onset of regrouping of large aglomerates fragments composed of α, β pitch components and PET, and destruction of interactions between smaller aggregates. Moreover, on the DSC curve for the composition containing 60 wt% of PET (Fig. 1c), an exothermic transition occurring at 148.3 °C, related probably to PET recrystallization was observed [20].
Addition of waste PET caused changes occurring in studied compositions as an effect of temperature, while the biggest changes were observed in decomposition temperature. The increase of PET addition into CTP caused the increase of decomposition temperature up to 397.1 °C. In the composition containing 10 wt% of waste PET, the dominant role was played by the pitch and so its decomposition was characteristic for this material. The courses of DSC curves for compositions containing ≥50 wt% of waste PET were similar to the course of DSC curve of PET. In these compositions the dominant role was played by waste polymer and as an effect of this, the decomposition course of these compositions was characteristic for PET.
The addition of waste PET significantly influenced the softening point of CTP. With the increase of PET in the compositions the softening point increased. For the composition containing 60 wt% of PET, the softening point compared to the unmodified pitch increased by 132 °C.
The changes of thermal properties of pitch-PET compositions were probably caused by the change of group composition of the bitumen. Addition of waste PET into CTP increased the amount of components insoluble in quinoline and toluene.
With the increase of concentration of waste PET in the compositions, the coking value decreased and so the yield of residue after high-temperature carbonization process. In particular, significant changes occurred for compositions containing 50 and 60 wt% of the waste.
Figure 2 presents DSC curves of selected AC obtained from CTP and pitch-polymer compositions. Table 2 contains the results of sorption properties and porous structure of obtained AC.
Analyzing the courses of DSC curves of AC, only endothermic transition occurring in the range of temperatures from 35 to 52 °C (enthalpy values from −6.100 to −18.260 J/g) was observed. This transition is probably related to desorption of water. With the increase of waste PET content in compositions, the temperature of this transition decreased.
It was also observed that the increase of waste PET addition into CTP caused the increase of development of S BET specific surface. Significant increase of this value, compared to activated carbon obtained from CTP, was obtained for AC prepared from compositions containing ≥45 wt% of waste PET.
From the comparison of the volume of micropores and mesopores, it can be concluded that AC obtained from pitch-PET compositions have microporous structure. Moreover, along with the increase of PET amount in compositions, the volume of micropores increased while the volume of mesopores increased only slightly.
Pitch-PF compositions
Figure 3 presents DSC curves of CTP, PF and selected pitch-PF compositions. Table 1 contains selected thermal and physicochemical properties of CTP and pitch-polymer compositions.
Based on DSC curves course for pitch-PF compositions (Fig. 3), it was found that transitions characteristic for both CTP and waste PF occurred in them as an effect of temperature, including:
-
glass transition of PF, occurring for respective compositions in the range of temperatures from 27.7 to 38.1 °C (heat capacity values from 0.014 to 0.261 J/(g K)),
-
endothermic transition related probably to PF melting, occurring for respective compositions in the range of temperatures from 58.6 to 70.0 °C (enthalpy values from −0.709 to −5.238 J/g),
-
endothermic transition, occurring for respective compositions in the range of temperatures from 101.0 to 103.6 °C (enthalpy values from −0.013 to −0.121 J/g), related probably to pitch melting,
-
endothermic transition, related probably to decomposition of samples. The onset of this transition for studied compositions occurred in the temperatures from 290.0 to 367.1 °C.
In DSC curves of pitch-PF compositions, the peak related to the transition from the brittle to the viscoelastic state was not observed. The biggest changes occurring in the compositions as an effect of temperature, compared to unmodified pitch, were observed for the decomposition temperature. Compositions containing ≥25 wt% of waste PF had the decomposition temperature 50–60 °C higher than CTP. Moreover, on the DSC curve for the composition containing 70 wt% of PF (Fig. 3), an exothermic transition occurring at 164.3 °C, related probably to condensation reaction of PF was observed [21].
Compositions containing PF had increased softening points, compared to CTP. Softening point increased with increasing content of waste PF. In the case of compositions containing ≥35 wt% of this component, the measurements of softening point was not possible because of the impossibility to melt them.
The changes of thermal properties of pitch-PF compositions were probably caused by the change of CTP group composition. Addition of waste PF into CTP caused significant increase of components insoluble in toluene amount, while the amount of components insoluble in quinoline decreased.
For the compositions containing PF, independently from the amount of polymer, coking values were similar to the value of CTP.
Figure 4 presents DSC curves of selected AC obtained from CTP and pitch-PF compositions. Table 2 contains the results of measurements of porous structure of these AC.
Analyzing the course of DSC curves of AC, an endothermic transition was observed in the range of temperatures from 38 to 49 °C (enthalpy values from −8.300 to −11.590 J/g). This transition is probably related to desorption of water. With the increase of waste PF content in compositions, the temperature of this transition increased, contrary to the case of AC obtained from pitch-PET compositions.
Analogically as in the case of AC obtained from pitch-PET compositions, the increase of the amount of waste PF added into CTP caused the increase of S BET specific surface development. Significant increase of this value, compared to activated carbon obtained from CTP, was obtained for AC prepared from compositions containing ≥45 wt% of waste PF. Moreover, with the increase of PF amount in compositions, the volume of mesopores and micropores increased.
Conclusions
The use of differential scanning calorimetry in the studies on pitch-polymer compositions made possible the determination of their phase transitions temperatures, and in the studies of AC, the determination of water desorption temperature. Results obtained in this study indicate that there is a possibility to obtain AC from pitch-polymer compositions with the use of waste PET and PF, preferably in the amounts ≥45 wt%.
The changes of thermal properties of CTP depended on the type and amount of applied waste polymer. Addition of waste PET into CTP increased its softening point and decomposition temperature, which was significantly affected by the amount of PET in compositions. Addition of waste PF caused the softening point increase of the CTP as well as decomposition temperature for compositions containing ≥25 wt% of waste polymer. The changes of thermal properties of pitch-polymer compositions were probably the effect of changes of group composition and mutual interactions between composition components.
The type and amount of waste polymer influenced also thermal properties and porous structure of AC obtained from pitch-polymer compositions:
-
in the case of AC prepared from pitch-PET compositions water desorption temperature decreased with the increase of PET amount in the pitch, with simultaneous improvement of sorption properties, specific surface development and increase of micropores volume, while the volume of mesopores increased only slightly,
-
in the case of AC obtained from pitch-PF compositions, water desorption temperature increased with increasing amount of PF in the pitch, with simultaneous improvement of sorption properties, specific surface development and increase of the volume of micropores and mesopores.
AC obtained from compositions containing ≥45 wt% of waste PET or PF exhibited the highest level of specific surface development and profitable porous structure.
References
Marsh H, Rodriguez-Reinoso F. Activated carbon. Amsterdam: Elsevier; 2006.
Guo J, Lua AC. Effect of heating temperature on the properties of chars and activated carbons prepared from oil palm stones. J Therm Anal Calorim. 2000;60:417–25.
Gonzalez T, Molina-Sabio M, Rodriguez-Reinoso F. Steam activation of olive stone chars, development of porosity. Carbon. 1994;32:1407–13.
Ciesińska W, Makomaski G, Zieliński J, Brzozowska T. Preparation of sorbents from selected polymers. Pol J Chem Technol. 2011;13:51–4.
Tóth A, Novák C, László K. The effect of ionic environment on the TG response of phenol loaded PET-based porous carbons. J Therm Anal Calorim. 2009;97:273–80.
Lorenc-Grabowska E, Gryglewicz G, Machnikowski J, Díez MA, Barriocanal C. Activated carbons from coal/pitch and polyethylene terephthalate blends for the removal of phenols from aqueous solutions. Energ Fuel. 2009;23:2675–83.
Machnikowski J, Rutkowski P, Díez MA. Co-treatment of novolac- and resole- type phenolic resins with coal-tar pitch for porous carbons. J Anal Appl Pyrolysis. 2006;76:80–7.
Machnikowski J, Grzyb B, Machnikowska H, Weber JV. Surface chemistry of porous carbons from N-polymers and their blends with pitch. Microporous Mesoporous Mater. 2005;82:113–20.
Zieliński J, Osowiecka B, Liszyńska B, Ciesińska W, Polaczek J, Kubica K. Benzo[a]pyrene in coal tar pitch: chemical conversion in situ by alkylation. Fuel. 1996;75:1543–8.
Zieliński J. The studying of structure and properties of bitumen–polymer compositions. Warsaw: Warsaw University of Technology; 1991.
Zieliński J, Piotrowska K, Polaczek J. Investigation on some properties of coal-tar pitch/polymer compositions and their possible applications. Polimery. 1993;38:537–42.
Brzozowska T, Zieliński J, Machnikowski J. Effect of polymeric additives to coal tar pitch on carbonization behavior and optical texture of resultant cokes. J Anal Appl Pyrolysis. 1998;48:45–58.
Ciesińska W, Zieliński J, Brzozowska T. Thermal treatment of pitch-polymer blends. J Therm Anal Calorim. 2009;95:193–6.
Ciesińska W, Makomaski G, Zieliński J, Brzozowska T, Pacewska B, Szychowski D. Studies on the preparation of microporous materials from the pitch-polymer compositions. Pol J Environ Stud. 2009;18:27–30.
Makomaski G, Zieliński J, Ciesińska W, Brzozowska T. Preparation and application of carbon sorbents from pitch-polymer compositions. In: Book of abstracts 9th international symposium on the characterisation of porous solids. Dresden; 2011. pp. 103.
Makomaski G, Zieliński J, Czepirski L, Ciesińska W, Brzozowska T. Study on the thermal properties of activated carbon preparation from the pitch-polymer compositions. In: Seminar materials of 10th international seminar on thermal analysis and calorimetry to the memory of Prof. Stanisław Bretsznajder. Płock; 2011. pp. 114–115.
Szychowski D, Pacewska B, Makomaski G, Zieliński J, Ciesińska W, Brzozowska T. Adsorption and DSC study of mineral-carbon sorbents obtained from coal tar pitch-polymer compositions. J Therm Anal Calorim. 2011. doi: 10.1007/s10973-010-1257-z.
Pacewska B, Szychowski D, Żmijewski T. Forum chemiczne 2000. Warsaw: Warsaw University of Technology; 2000.
Wang C, Zhang Z, Mai K. Preparation, non-isothermal crystallization, and melting behavior of β-nucleated isotactic polypropylene/poly(ethylene terephthalate) blends. J Therm Anal Calorim. 2011;106:895–903.
Sauer B, Kampert W, McLean R, Carcia P. TMDSC and atomic force microscopy studies of morphology and recrystallization in polyesters including oriented films. J Therm Anal Calorim. 2000;59:227–43.
Park B, Wang X. Thermokinetic behavior of powdered phenol-formaldehyde (PPF) resin. Thermochim Acta. 2005;433:88–92.
Acknowledgements
The study was financed from National Science Centre (NN209763640).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Makomaski, G., Ciesińska, W. & Zieliński, J. Thermal properties of pitch-polymer compositions and derived activated carbons. J Therm Anal Calorim 109, 767–772 (2012). https://doi.org/10.1007/s10973-012-2373-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2373-8