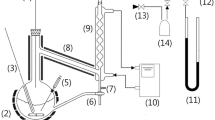
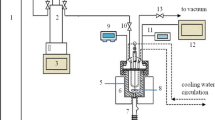
Abstract
The thermodynamic evaluation of the experimental vapor–liquid equilibrium (VLE) data obtained at 760 mmHg in a recirculatory still, is presented for the binary system formed by diethylenimide oxide with benzene. The experimental VLE data were checked for thermodynamic consistency and reduced to the binary parameters calculated from three activity coefficients models.



Similar content being viewed by others
References
Palczewska-Tulinska M, Choliński J, Szafranski A, Wyrzykowska-Stankiewicz D. Isobaric vapor liquid equilibrium in three binary systems involving morpholine. Fluid Phase Equilib. 1980;5:113–29.
Pettenati C, Alessi P, Fermeglia M, Kikic I. Vapor liquid equilibrium data for systems containing morpholine. Fluid Phase Equilib. 1990;54:81–91.
Wu HS, Locke WE, Sandler SJ. Isothermal vapor–liquid equilibrium of binary mixtures containing morpholine. J Chem Eng Data. 1991;36:127–30. doi:10.1021/je00001a037.
Marrufo B, Sanchotello M, Loras S. Isobaric VLE for binary and ternary mixtures with cyclohexane, ciclohexene and morpholine at 100 kPa. Fluid Phase Equilib. 2010;296:178–83.
Palczewska-Tulinska M, Wyrzykowska-Stankiewicz D, Szafranski A. Isobaric vapor liquid equilibrium in methanol and benzene–morpholine systems. Fluid Phase Equilib. 1994;100:241–52.
Drebushchak V. Concepts against mathematics, self-inconsistency in thermodynamic evaluation. J Therm Anal Calorim 2011; doi:10.1007/s1097301008452.
Timmermans J. Physico-chemical constants of pure organic compounds. New York: Elsevier Publishing Co.; 1950.
Gothard FA, Brassat F. Rev Chim (Bucharest Rom). 1969; 20: 213–19.
Hayden JG, O’Connell JP. A generalized Method for predicting second virial coefficients. Ind Eng Chem Process Des Dev. 1975;14:209–16.
Rackett HG. Equation of state for saturated liquids. J Chem Eng Data. 1970;15:514–7.
Kojima K, Moon HM, Ochi K. Thermodynamic consistency test of vapor–liquid equilibrium data: methanol–water, benzene–cyclohexane and ethyl methyl ketone–water. Fluid Phase Equilib. 1990;56:269–80.
Simoiu L, Trandafir I, Popescu G. New thermodynamic consistency test for isobaric VLE data. J Therm Anal Calorim. 1998;52:1023–35.
Dodescu G, Toma M. Metode de Calcul Numeric. Bucuresti: Ed Didactica; 1976.
Dorn S, Cracken D. Numerical methods. Bucuresti: Ed Technica; 1972.
Gluck A. Metode statistice in chimie. Ed Technica: Bucuresti; 1971.
Wilson GM. Vapor liquid equlibrium XI. A new expression for the excess free energy of mixing. J Am Chem Soc. 1964;86:127–30.
Renon H, Prausnitz JM. Local composition in thermodynamic excess functions for liquid mixtures. AIChE J. 1968;14:135–44.
Gothard F. Predicting liquid–vapor equilibria by a model of the interaction equilibrium in restrained molecular systems. Ind Eng Chem Fundam. 1985;24:330–9.
Panaitescu G. Maximum likelihood principle extended to generate selection criteria for equations of sate/or data. Rev Roum Chim. 1991;36:1195–2001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simoiu, AM., Iacob, A. Thermodynamic study of the vapor–liquid equilibrium (VLE) data of the mixture formed by diethylenimide oxide with benzene. J Therm Anal Calorim 110, 329–334 (2012). https://doi.org/10.1007/s10973-012-2345-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2345-z