Abstract
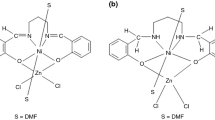
To compare thermal stability of Co(II), Zn(II), and Cd(II) complexes with 4-CHO-5-MeIm, the two compounds of formula [MnL2(NO3)2] and [NiL3](NO3)2 have been prepared and structurally characterized. Elemental analysis and spectroscopic studies have confirmed a bidentate fashion of coordination of the ligand to Mn(II) and Ni(II) ions. IR and Raman spectra indicate that there are different coordination modes of the NO3 − in compounds: non-coordinated and coordinated. The decomposition process of the studied complexes in nitrogen and argon (Ni(II) complex) atmosphere proceeds in three main stages, except Zn(II) complex, in temperature range 353–1163 K. The final products of decomposition are CoO, MnO, Cd, ZnN4, NiN3. In addition, we have to admit that the different coordination mode of the NO3 − ions in complexes: non-coordinated (in the (1), (4), and (5)) and coordinated (in the (2) and (3)) correlate with its thermal behavior. Thus, temperature ranges of its decompositions are observed: below 533 K and above 533 K, respectively. In Co(II), Mn(II), and Cd(II) complexes the fragments of N-donor atom-containing ligands decompose in the last stages, contrary to Zn(II) and Ni(II) compounds, in which metal ion surrounded by N atoms remains until the end. The course of pyrolysis and molecular structure of the complexes lead to the same conclusion about the strength of metal–ligand bonds. On the basis of obtained results, it is concluded that the thermal stability of the studied compounds follows the order: (1) < (5) < (2) < (3) < (4).




Similar content being viewed by others
References
Barszcz B, Hodorowicz S, Stadnicka K, Jabłońska-Wawrzycka A. A comparison of the coordination geometries of some 4-methylimidazole-5-carbaldehyde complexes with Zn(II), Cd(II) and Co(II) ions in the solid state and aqueous solution. Polyhedron. 2005;24:627–37.
Gockel P, Vogler R, Gelinsky M, Meißner A, Albrich H, Vahrenkamp H. Zinc complexation of cyclic dipeptides containing cysteine and/or histidine. Inorg Chim Acta. 2001;323:16–22.
Adult DS. Zinc coordination sphere in biochemical zinc sites. Biometals. 2001;14:271–313.
Adult DS. The ins and outs of biological zinc sites. Biometals. 2009;22:141–8.
Christianson DW, Lipscomb WN. X-ray crystallographic investigation of substrate binding to carboxypeptidase A at subzero temperature. Proc Natl Acad Sci USA. 1986;83:7568–72.
Christianson DW, Cox JD. Catalysis by metal-activated hydroxide in zinc and manganese metalloenzymes. Annu Rev Biochem. 1999;68:33–57.
Müller B, Vahrenkamp H. Zinc complexes of chelating aldehydes. Eur J Inorg Chem 1999;137–44.
Hoog JO, Hedberg JJ, Stromberg P, Svensson S. Mammalian alcohol dehydrogenase—functional and structural implications. J Biomed Sci. 2001;8:71–6.
Dowling C, Parkin G. Elaboration of the bis(pyrazolyl)hydroborato ligand [BpBut, Pri] into the NNO donor ligand, [(MeO)BpBut, Pri]: structural characterization of a complex in which the [(MeO)BpBut, Pri] ligand models the binding of zinc to the peptide backbone in thermolysin. Polyhedron. 1996;15:2463–5.
Adams H, Bradshaw D, Fenton DE. A co-ordination number asymmetric dinuclear zinc(II) complex of an unsymmetrical compartmental proligand. Polyhedron. 2002;21:1957–60.
Gunnlaugsson T, Nieuwenhuyzen M, Nolan C. Synthesis, X-ray crystallographic, spectroscopic investigation and cleavage studies of HPNP by simple bispyridyl iron, copper, cobalt, nickel and zinc complexes as artificial nucleases. Polyhedron. 2003;22:3231–42.
Weder JE, Dillon CT, Hambley TW, Kennedy BJ, Lay PA, Biffin JR, Regtop HL, Davies NM. Copper complexes of non-steroidal anti-inflammatory drugs: an opportunity yet to be realized. Coord Chem Rev. 2002;232:95–126.
Milanino R, Mauro U, Marrella M, Pasqualicchio M, Gasperini R, Velo G. In: Berthon G, editor. Handbook of metal–ligand interactions in biological fluids. New York: Marcel Decker; 1995. p. 886.
Casella L, Gullotti M. Dioxygen activation by biomimetic dinuclear complexes. In: Karlin KD, Tyeklar Z, editors. Bioinorganic chemistry of copper. New York: Chapman & Hall; 1993. p. 292–305.
Liang HC, Dahan M, Karlin KD. Dioxygen-activating bio-inorganic model complexes. Curr Opin Chem Biol. 1999;3:168–75.
Mahadevan V, Gebbink RJMK, Stack TDP. Biomimetic modeling of copper oxidase reactivity. Curr Opin Chem Biol. 2000;4:228–34.
Zhang F, Shen L. Synthesis, characterization and SOD activity of manganese(II) complexes with aza-macrocyclic ligand. J Chem Crystallogr. 2010;40:681–5.
Lee H, Park W, Lim D. Synthesis and SOD activity of manganese complexes of substituted pyridino pentaaza macrocycles that contain axial auxiliary. Bioorg Med Chem Lett. 2010;20:2421–4.
Singh UP, Tyagi P, Upreti S. Manganese complexes as models for manganese-containing pseudocatalase enzymes: Synthesis, structural and catalytic activity studies. Polyherdon. 2007;26:3625–32.
Jarenmark M, Carlsson H, Nordlander E. Asymmetric dinuclear metal complexes as models for active sites in hydrolases and redox enzymes. C R Chimie. 2007;10:433–62.
Barszcz B, Głowiak T, Jabłońska-Wawrzycka A. Ligation of alkoxymethylimidazoles towards cadmium(II) and zinc(II). X-ray, spectroscopic, thermal and potentiometric investigation. Trans Met Chem. 2005;30:221–8.
Barszcz B, Hodorowicz S, Jabłońska-Wawrzycka A, Stadnicka K. Synthesis, X-ray structure and spectroscopic investigation of eight-coordinate cadmium (II) complex. J Coord Chem. 2005;58:203–8.
Jabłońska-Wawrzycka A, Zienkiewicz M, Masternak J, Rogala P. Novel eight-coordinated Cd(II) complexes with two homologous pyridine alcohols. Crystal structure, spectroscopic and thermal properties. J Mol Struct 2012;1012:97–104.
Barszcz B, Jabłońska-Wawrzycka A, Stadnicka K, Hodorowicz S. The synthesis and structural characterization of novel zinc and cadmium complexes of chelating alcohol. Inorg Chem Commun. 2005;8:951–4.
Barszcz B, Masternak J, Surga W. Thermal properties of Ca(II) and Cd(II) complexes of pyridinedicarboxylates. J Therm Anal Cal. 2010;101:633–9.
Barszcz B, Hodorowicz M, Jabłońska-Wawrzycka A, Masternak J. Comparative study on Cd(II) and Ca(II) model complexes with pyridine-2,3-dicarboxylic acid: Synthesis, crystal structure and spectroscopic investigation. Polyhedron. 2010;29:1191–200.
Jabłońska-Wawrzycka A, Barszcz B, Stadnicka K. Similarities and differences of thermal behaviour of 2-hydroxymethylbenzimidazole complexes with Zn(II) and Cd(II) ions. J Therm Anal Cal. 2010;101:463–9.
Bal W, Kozlowski H, Kasprzak KS. Molecular models in nickel carcinogenesis. J Inorg Biochem. 2000;79:213–8.
Minang JT, Ahlborg N, Troye-Blomberg M. A simplified ELISpot assay protocol used for detection of human interleukin-4, interleukin-13 and interferon-γ production in response to the contact allergen nickel. Exogenous Dermatol. 2003;2:306–13.
Jakobson E, Masjedi K, Ahlborg N. Cytokine production in nickel-sensitized individuals analysed with enzyme-linked immunospot assay: possible implication for diagnosis. Br J Dermatol. 2002;147:442–9.
Toebak MJ, Pohlmann PR, Sampat-Sardjoepersad SC. CXCL8 secretion by dendritic cells predicts contact allergens from irritants. Toxicol In Vitro. 2006;20:117–24.
Barszcz B, Głowiak T, Jezierska J. Crystal and molecular structures of eight-coordinate (CuN4O4) and six-coordinate (CuN4O2) Cu(II) complexes with 4-methyl-5-imidazole-carboxaldehyde or 1-benzyl-2-hydroxymethylimidazole, respectively. Polyhedron. 1999;18:3713–21.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds. 6th ed. Hoboken: Wiley; 2009.
Lever ABP, Mantovani E, Ramaswamy BS. Infrared combination frequencies in coordination complexes containing nitrate groups in various coordination environment. A probe for the metal-nitrate interaction. Can J Chem. 1971;49:1957–64.
Khalil MMH, Ismail EH, Abdel Azim S, Souaya ER. Synthesis, characterization, and thermal analysis of ternary complexes of nitrilotriacetic acid and alanine or phenylalanine with some transition metals. J Therm Anal Calorim. 2010;101:129–35.
Soliman MH, Mohamed GG, Mohamed EA. Metal complexes of fenoterol drug. Preparation, spectroscopic, thermal, and biological activity characterization. J Therm Anal Calorim. 2010;99:639–47.
Doğan F, Ulusoy M, Öztürk ÖF, Kaya İ, Salih B. Thermal studies of Co(II), Ni(II) and Cu(II) complexes of N, N’-bis(3,5-di-t-butylsalicylidene)ethylenediamine. J Therm Anal Cal. 2009;96:267–76.
Powder Diffraction File, JCPDS: ICDD, 1601 Park Lane, Swarthmore, PA 19081, Data 1990, File No 9–402.
Mazur M, Gontarz Z. Reactions of manganese oxides with K2S2O7. J Therm Anal Calorim. 2010;100:993–8.
Dose WM, Donne SW. Kinetic analysis of γ-MnO2 thermal treatment. J Therm Anal Calorim. 2011;105:113–22.
Powder Diffraction File, JCPDS: ICDD, 1601 Park Lane, Swarthmore, PA 19081, Data 1990, File No 7–230.
Singh G, Singh CP, Frohlich R. Preparation, characterization and thermolysis of metal nitrate complexes with 4,4′-bipyridine. J Therm Anal Cal. 2006;85:425–31.
Acknowledgements
The authors are grateful to Joanna Masternak MSc, for help during the thermal work. European Union Project 8.2.1/POKL/2009 partly supported this study (M. Zienkiewicz). The opportunity of making the Raman spectra in the Structural Laboratory of the Jan Kochanowski University are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jabłońska-Wawrzycka, A., Zienkiewicz, M., Barszcz, B. et al. Thermoanalytical study of selected transition bivalent metal complexes with 5-carbaldehyde-4-methylimidazole. J Therm Anal Calorim 109, 735–743 (2012). https://doi.org/10.1007/s10973-012-2329-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2329-z