Abstract
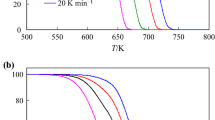
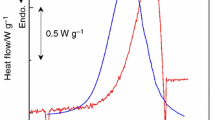
The kinetics of thermal decomposition of NH4CuPO4·H2O was studied using isoconversional calculation procedure. The iterative isoconversional procedure was applied to estimate the apparent activation energy E a; the values of apparent activation energies associated with the first stage (dehydration), the second stage (deamination), and the third stage(condensation) for the thermal decomposition of NH4CuPO4·H2O were determined to be 117.7 ± 7.7, 167.9 ± 8.4, and 217.6 ± 45.5 kJ mol−1, respectively, which demonstrate that the third stage is a kinetically complex process, and the first and second stages are single-step kinetic processes and can be described by a unique kinetic triplet [E a, A, g(α)]. A new modified method of the multiple rate iso-temperature was used to define the most probable mechanism g(α) of the two stages; and reliability of the used method for the determination of the kinetic mechanism were tested by the comparison between experimental plot and model results for every heating rate. The results show that the mechanism functions of the two stages are reliable. The pre-exponential factor A of the two stages was obtained on the basis of E a and g(α). Besides, the thermodynamic parameters (ΔS ≠, ΔH ≠, and ΔG ≠) of the two stages were also calculated.





Similar content being viewed by others
References
Vasovic DD, Stojakovic DR. Metal phosphate preparation using boron phosphate. Mater Res Bull. 1997;32:779–84.
Chen J, Natarajan S, Thomas JM, Jones RH, Hursthouse MB. A novel open-framework cobalt phosphate containing a tetrahedrally coordinated cobalt(II) center: CoPO4·0.5C2H10N2. Angew Chem Int Ed. 1994;33:639–40.
Gier TE, Stucky GD. Low-temperature synthesis of hydrated zinco(beryllo)-phosphate and arsenate molecular sieves. Nature. 1991;349:508–10.
Ng HY, Harrison WTA. Monoclinic NaZnPO4-ABW, a new modification of the zeolite ABW structure type containing elliptical eight-ring channels. Micropor Mesopor Mater. 1998;23:197–202.
Harrison WTA, Gier TE, Nicol JM, Stucky GD. Tetrahedral-framework lithium zinc phosphate phases: location of light-atom positions in LiZnPO4·H2O by powder neutron diffraction and structure determination of LiZnPO4 by ab initio methods. J Solid State Chem. 1995;114:249–57.
Jensen TR. A new polymorph of LiZnPO4·H2O: synthesis, crystal structure and thermal transformation. J Chem Soc Dalton Trans. 1998;18:2261–6.
Bu XH, Gier TE, Stucky GD. A new polymorph of lithium zinc phosphate with the cristobalite-type framework topology. J Solid State Chem. 1998;138:126–30.
Bensalem A. Synthesis and characterization of a new layered lithium zinc phosphate hydrate. J Solid State Chem. 2001;162:29–33.
Jensen TR, Hazell RG, Nørlund Christensen A, Hanson JC. Hydrothermal synthesis of lithium zinc phosphates: structural investigation of twinned α-Li4Zn(PO4)2 and a high temperature polymorph β-Li4Zn(PO4)2. J Solid State Chem. 2002;166:341–51.
Chan TS, Liu RS, Baginskiy I. Synthesis, crystal structure, and luminescence properties of a novel green-yellow emitting phosphor LiZn1−x PO4:Mn x for light emitting diodes. Chem Mater. 2008;20:1215–7.
Pujana A, Pizarro JL, Lezama L, Gon˜i A, Arriortua MI, Rojo T. Synthesis, crystal structure, and magnetic properties of NH4CuPO4·H2O. J Mater Chem. 1998;8:1055–60.
Koo HJ, Whangbo MH. On the correct spin lattice for the spin-gapped magnetic solid NH4CuPO4·H2O. J Solid State Chem. 2008;181:276–81.
Liao S, Chen ZP, Tian XZ, Wu WW. Synthesis and regulation of α-LiZnPO4·H2O via a solid state reaction at low-heating temperatures. Mater Res Bull. 2009;44:428–31.
Liao S, Tian XZ, Chen X, Wu WW, Liang YG. Selective synthesis of a hexagonal Co(II)-substituted sodium zincophosphate via a simple and novel route. Chin J Chem. 2010;28:50–4.
Liao S, Chen ZP, Liu G, Tian XZ, Wang TS, Wu WW. Preparation of ammonium cerium phosphate via low-heating solid state reaction and its catalysis for benzyl acetate synthesis. Chin J Chem. 2010;28:378–81.
Wu WW, Fan YJ, Wu XH, Liao S, Li SS. Preparation via solid-state reaction at room temperature and characterization of layered nanocrystalline NH4MnPO4·H2O. J Phys Chem Solid. 2009;70:584–7.
Wu XH, Wu WW, Liao S, Fan YJ, Li SS. Preparation via solid-state reaction at room temperature and characterization of layered nanocrystalline KMnPO4·H2O. J Alloy Compd. 2009;479:541–4.
Liu C, Wu XH, Wu WW, Cai JC, Liao S. Preparation of nanocrystalline LiMnPO4 via a simple and novel method and its isothermal kinetics of crystallization. J Mater Sci. 2011;46:2474–8.
Wu WW, Wu XH, Lai SB, Liao S. Non-isothermal kinetics of thermal decomposition of NH4ZrH(PO4)2·H2O. J Therm Anal Calorim. 2011;104:685–91.
Wu XH, Wu WW, Lai SB, Cui XM, Liao S. Kinetics and thermodynamics of thermal decomposition of NH4NiPO4·6H2O. J Therm Anal Calorim. 2011;103:805–12.
Wu XH, Wu WW, Cui XM, Liao S. Preparation of nanocrystalline BiFeO3 via a simple and novel method and its kinetics of crystallization. J Therm Anal Calorim. 2011; doi:10.1007/s10973-011-1483-z.
Chen ZP, Chai Q, Liao S, He Y, Wu WW, Li B. Preparation of LiZnPO4·H2O via a novel modified method and its non-isothermal kinetics and thermodynamics of thermal decomposition. J Therm Anal Calorim. 2011; doi:10.1007/s10973-011-1799-8.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Genieva SD, Vlaev LT, Atanassov AN. Study of the thermooxidative degradation kinetics of poly(tetrafluoroethene) using iso-conversional calculation procedure. J Therm Anal Calorim. 2010;99:551–61.
Li LQ, Chen DH. Application of iso-temperature method of multiple rate to kinetic analysis. Dehydration for calcium oxalate monohydrate. J Therm Anal Calorim. 2004;78:283–93.
Guan CX, Shen YF, Chen DH. Comparative method to evaluate reliable kinetic triplets of thermal decomposition reactions. J Therm Anal Calorim. 2004;76:203–16.
Gao Z, Amasaki I, Nakada M. A description of kinetics of thermal decomposition of calcium oxalate monohydrate by means of the accommodated Rn model. Thermochim Acta. 2002;385:95–103.
Gao X, Dollimore D. The thermal decomposition of oxalates: Part 26. A kinetic study of the thermal decomposition of manganese(II) oxalate dehydrate. Thermochim Acta. 1993;215:47–63.
Ozawa TA. New method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Paik P, Kar KK. Kinetics of thermal degradation and estimation of lifetime for polypropylene particle: effect of particle size. Polym Degrad Stab. 2008;93:24–35.
Flynn JH. The ‘temperature integral’—its use and abuse. Thermochim Acta. 1997;300:83–92.
Seo DK, Park SS, Hwang J, Yu TU. Study of the pyrolysis of biomass using thermo-gravimetric analysis (TGA) and concentration measurements of the evolved species. J Anal Appl Pyrolysis. 2010;89:66–73.
Seo DK, Park SS, Kim YT, Hwang J, Yu TU. Study of coal pyrolysis by thermo-gravimetric analysis (TGA) and concentration measurements of the evolved species. J Anal Appl Pyrolysis. 2011;92:209–16.
Chrissafis K, Paraskevopoulos KM, Papageorgiou GZ, Bikiaris DN. Thermal decomposition of poly(propylene sebacate) and poly(propylene azelate) biodegradable polyesters: evaluation of mechanisms using TGA, FTIR and GC/MS. J Anal Appl Pyrolysis. 2011;92:123–30.
Chrissafis K. Kinetics of thermal degradation of polymers. Complementary use of iso-conversional and model-fitting methods. J Therm Anal Calorim. 2009;95:273–83.
Cadenato A, Morancho JM, Fernandez-Francos X, Salla JM, Ramis X. Comparative kinetic study of the non-isothermal thermal curing of bis-GMA/TEGDMA systems. J Therm Anal Calorim. 2007;89:233–44.
Su TT, Jiang H, Gong H. Thermal stabilities and the thermal degradation kinetics of poly(ε-caprolactone). Polymer-Plastics Technol Eng. 2008;47:398–403.
Senum GI, Yang RT. Rational approximations of the integral of the Arrhenius function. J Therm Anal. 1977;11:445–7.
Boonchom B, Puttawong S. Thermodynamics and kinetics of the dehydration reaction of FePO4·2H2O. Phys Bull. 2010;405:2350–5.
Vlaev L, Nedelchev N, Gyurova K, Zagorcheva M. A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. J Anal Appl Pyrolysis. 2008;81:253–62.
Boonchom B. Kinetics and thermodynamic properties of the thermal decomposition of manganese dihydrogenphosphate dihydrate. J Chem Eng Data. 2008;53:1533–8.
Vlaev LT, Nikolova MM, Gospodinov GG. Non-isothermal kinetics of dehydration of some selenite hexahydrates. J Solid State Chem. 2004;177:2663–9.
Boonchom B. Kinetic and thermodynamic studies of MgHPO4·3H2O by non-isothermal decomposition data. J Therm Anal Calorim. 2009;98:863–71.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 21161002), the Key laboratory of new processing technology for nonferrous metals and materials, Ministry of Education, Guangxi University (No. GXKFZ-02), the Guangxi Natural Scientific Foundation of China (Grant No. 0991108 and. 0832111), and the Guangxi Science and Technology Agency Research Item of China (Grant No. 0895002–9).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, Y., Liao, S., Chen, Z. et al. Application of isoconversional calculation procedure to non-isothermal kinetics study. J Therm Anal Calorim 111, 313–321 (2013). https://doi.org/10.1007/s10973-012-2306-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2306-6