Abstract
The enthalpies of solution of l-α-aspartic acid, l-α-glutamic acid, l-α-arginine, l-α-lysine, and l-α-histidine have been measured in aqueous ethanol solutions at 298.15 K. From the obtained experimental results, the standard enthalpies of solution of amino acids in water–ethanol solutions have been determined. These data were used to calculate the heterogeneous enthalpic pair interaction coefficients based on McMillan–Mayer’s formalism. These values were interpreted in the terms of the ionic or no polar effect of the side chains of l-α-amino acids on their interactions with a molecule of ethanol in water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many research centers carry out studies on factors that exert influence on the stability of the native structure of proteins, processes of their folding and interactions with various components of body fluids. The complex structure of biomacromolecules poses difficulties in the interpretation of the processes, in which they participate. Therefore, simple model systems such as l-α-amino acids [1–3] or small peptides [4–6] are often used for such investigations. Moreover, every living organism contains a constant stock of free natural amino acids, which participate in life processes, interact with the components of intracellular and extracellular fluids.
The aim of our study was to examine the interactions between l-α-amino acids and ethanol proceeding in aqueous solutions. Ethanol occurs in the organisms of yeast and many bacteria as a product of glucose pathway. It is supplied to animal and human organisms as a component of numerous foods, pharmaceutical and cosmetic products. Moreover, ethanol is of interest as a compound possessing two different functional groups with respect to the affinity to water: polar hydroxyl group and non-polar ethyl group.
Based on the values of the solution enthalpy of selected amino acids in aqueous ethanol solutions obtained by the calorimetric method, enthalpic interaction coefficients derived from modified [7, 8] McMillan–Mayer’s formalism [9] were determined. These coefficients describe the energetic effects of the interactions between the molecules under investigation proceeding with the competitive co-contribution of water molecules.
Experimental section
Materials
l-α-Aspartic acid (Asp), l-α-glutamic acid (Glu), l-α-arginine (Arg), l-α-lysine (Lys), and l-α-histidine (His) (all mass fraction purity >99%, Fluka) were dried vacuum at 320 K; ethanol (99.8% POCh Poland) was purified by standard methods [10]. The water used in the experiments was deionized, distilled twice, and degassed.
Calorimetric measurements
The enthalpies of solution were measured in water and in aqueous solutions of ethanol (EtOH) using an isoperibol calorimeter [11]. The temperature sensitivity was about 0.00004 K and the temperature stability of the thermostat was better than 0.002 K. The uncertainties in the measured enthalpies did not exceed ±0.5% of the measured value. The examined aqueous solutions containing from 0 to 5 mol of ethanol in 1 kg of water and the aqueous solutions of amino acids (A) (0.001 to 0.005) mol (A) kg−1 (solvent) were prepared by weight using Mettler AE 240 balance within the precision ±0.00001 g (buoyancy corrections to the weights were (±0.00002 g). The standard enthalpies of solution of l-α-amino acids (A) were determined by the linear extrapolation to zero amino acids concentration of the results obtained for eight to ten independent measurements within the investigated range of the amino acids concentration.
Results and discussion
The determined standard solution enthalpies of the l-α-amino acids (A) in ethanol (EtOH) and water (W) mixtures \( \{ \Updelta_{\text{sol}} H_{\text{m}}^{\infty } ({\text{W}} + {\text{EtOH}})\} \), together with their standard deviations, are presented in Table 1.
The values of the standard enthalpies of solution of all the amino acids tested increase with ethanol concentration in water (Table 1). Considering the changes in thermal effects accompanying the dissolution of amino acids in aqueous solution of ethanol, one should take into account the properties of this mixed solvent. Within the concentration range investigated, the addition of ethanol to water brings about the structure reinforcement of the mixture formed [12, 13]. The addition of amino acid to a solvent with a more developed structure such as water/ethanol mixture requires more energy to overcome the reinforced interactions existing in this mixture. Consequently, the dissolution process of amino acids becomes more endothermic.
Information about the interactions proceeding in aqueous solutions between the zwitterions of natural amino acids under investigation and ethanol molecule can be provided by the analysis of the enthalpic interaction coefficients of ethanol molecule–amino acid zwitterion pairs.
The values of standard dissolution enthalpies were used also to obtain the enthalpic heterogeneous pair interaction coefficients by the equation proposed by Desnoyers [14]:
where \( \Updelta_{sol} {\text{H}}_{m}^{\infty } (W) \) is the standard enthalpies of solution of l-α-amino acids in water (see Table 1) h A,EtOH is the heterogeneous enthalpic pair interaction coefficient between zwitterions of amino acids and ethanol molecule (see Table 2), h A,EtOH,EtOH is the enthalpic triplet interaction coefficient (see Table 2), and m EtOH denotes the molality concentration of ethanol in water (mol kg−1). The interpretation of the triplet interaction coefficient is obscured by the fact that they also contain some contributions from the pairwise interaction terms and they are not discussed in this article.
The values of the enthalpic pair interaction coefficients between amino acids zwitterions and ethanol molecule in water (Table 2) describe the energetic effects of the interactions between the molecules investigated proceeding with the competitive co-contribution of water molecules. The enthalpic pair interaction coefficients include the energetic co-contribution of both the water molecules creating hydration layers around polar or ionic groups and the water molecules constituting the hydrophobic sheaths around non-polar groups.
The direct interactions between the hydroxyl group of ethanol molecule and the zwitterion head of NH3 +CHCOO− as well as polar or ionic side substituents of amino acids (exothermic process) are possible if some water molecules are removed from their hydration layer (endothermic process). This endothermic process of dehydration is intensified by the hydrophobic effects induced by the ethyl group of ethanol as well as the non-polar groups of amino acid side chain. Water molecules surrounding non-polar groups reinforce the interactions between themselves owing to the cooperativeness of hydrogen bonds. This effect is transferred onto the water molecules of hydration layers of polar or ionic groups. Thereby the removal of some water molecules from hydration sheaths requires an increased energy input (increase in the endothermic process).
The enthalpic pair interaction coefficients, h (A,EtOH) (Table 2), of ethanol and l-α-amino acids (l-α-aspartic acid, l-α-glutamic acid, l-α-arginine, l-α-lysine, and l-α-histidine) possessing ionic substituents are more positive than the value of the enthalpic coefficient for glycine, h (Gly,EtOH) [15]. The high positive values of these coefficients point to the endothermic summary effect of the interaction between the zwitterions investigated and ethanol molecule in water. The positive values of enthalpic coefficients, h (A,EtOH), testify to the predominating effects of partial dehydration of ionic groups in amino acid side chains over the direct interaction between polar groups in the molecules are under discussion.
The calculated enthalpic pair interaction coefficients, h (A,EtOH), describing the interactions of ethanol with l-α-aspartic acid, l-α-glutamic acid, l-α-arginine, l-α-lysine, and l-α-histidine are surprisingly high in comparison with the values of the enthalpic coefficients of urea interaction with the zwitterions of the amino acids under discussion, h (A,U) [2] (see Table 2). These differences seem to be due to the specific structure of water–ethanol mixtures. The structure of ethanol makes it possible for it to be very well accommodated in the elastic three-dimensional network of hydrogen bonds of water [12, 13] bringing about the reinforcement of water structure [16]. The zwitterion heads of NH3 +CHCOO− and polar or ionic side substituents incorporated into the water–ethanol mixtures interact stronger with the surrounding water molecules than with the less accessible hydroxyl group of ethanol. Thereby the direct interactions between polar or ionic groups of l-α-amino acids and the hydroxyl group of ethanol make a smaller than expected exothermic contribution to the global effect of interactions that describe the enthalpic pair interaction coefficients. A crucial contribution to the values of enthalpic coefficients, h (A,EtOH), is made by the endothermic processes of dehydration of zwitterion head, polar or ionic groups in amino acid side chains, and the hydroxyl group of ethanol molecule. Therefore, the total effect described with the enthalpic pair interaction coefficients, h (A,EtOH), is so strongly endothermic.
The substitution of hydrogen atom in the side chain of l-α-alanine (h (Ala,EtOH) = 658 J kg mol−2) [15] or l-α-aminobutyric acid (h (Aba,EtOH) = 820 J kg mol−2) [17] with carboxylic group leads to the increase of the values of the enthalpic pair interaction coefficients, h (A,EtOH) (Table 2).
The dependence observed is the reverse of that shown in the system with urea, for which the enthalpic interaction coefficients of amino acid–urea pairs, h (A,U), in the case of l-α-alanine (h (A,U) = −238.2 J kg mol−2) [2] and l-α-aminobutyric acid (h (A,U) = −185 J kg mol−2) [18], have more positive values than those determined for l-α-aspartic acid or l-α-glutamic acid [2] (Table 2).
Comparing the values of enthalpic coefficients, h (A,EtOH), of l-α-aspartic acid and l-α-glutamic acid, one can observe the expected endothermic contribution induced by the presence of non-polar CH2 group: h (Asp,EtOH) < h (Glu,EtOH)
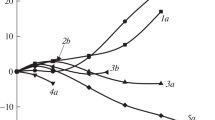
The values of the enthalpic coefficients of the heterogeneous amino acid–ethanol interactions, h (A,EtOH), were correlated with the values of the enthalpic coefficients of amino acid–urea interactions, h (A,U) [2] (see Table 3). This correlation has approximately a linear course (R 2 = 0.952, see Fig. 1). This testifies to similar contributions made by the partial effects of the dehydration of solvation layers in polar and ionic groups of the zwitterions of l-α-amino acids and direct interaction between discussed molecules to the total effects of interactions proceeding in examined systems it is: natural amino acids with ethanol (h (A,EtOH)) and natural amino acids with urea molecule (h (A,U)) in aqueous solution.
Relationship between the enthalpic pair interaction coefficients h (A,U) [2] of amino acids zwitterions–urea molecule in water and the enthalpic pair interaction coefficients h (A,EtOH) of amino acids zwitterions–ethanol molecule in water
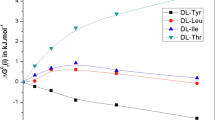
The values of the enthalpic pairs coefficients h (A,EtOH) were also compared with lipophilicity scale of amino acids calculated by Eisenberg and McLachlan [19] (see Table 3). The obtained graph (Fig. 2) displays dependence (R 2 = 0.855) between the lipophilicity parameters ΔG R and enthalpic pair interaction coefficients of amino acids and ethanol molecule h (A,EtOH). The observed dependences suggest, as well as above correlation, the similar contributions of the amino acid side substituents to the variability of numerical values of the correlated parameters that describe the behavior of the system in aqueous medium.
Relationship between the hydrophobicity parameters of amino acids ΔG R [19] and the enthalpic pair interaction coefficients h (A,EtOH) of amino acids zwitterions–ethanol molecule in water
Abbreviations
- h A,EtOH :
-
The heterogeneous enthalpic pair interaction coefficient between zwitterions of amino acids and ethanol molecule
- h A,EtOH,EtOH :
-
The enthalpic triplet interaction coefficient
- \( \Updelta_{sol} {\text{H}}_{m}^{\infty } (W) \) :
-
Standard enthalpies of solution of l-α-amino acids in water (W)
- \( \Updelta_{\text{sol}} H_{\text{m}}^{\infty } ({\text{W}} + {\text{EtOH}}) \) :
-
Standard solution enthalpies of the l-α-amino acids (A) in ethanol (EtOH) and water (W) mixtures
- \( m_{\text{EtOH}} \) :
-
Molal concentration of ethanol in water (mol kg−1)
References
Palecz B. Enthalpies of solution and dilution of some L-α-amino acids in water at 298.15 K. J Therm Anal Calorim. 1998;54:257–63.
Palecz B. Enthalpic pair interaction coefficient between zwitterions of L-α-amino acids and urea molecule as a hydrophobicity parameter of amino acid side chains. J Am Chem Soc. 2005;127:17768–71.
Palecz B, Dunal J. Interaction between several L-α-amino acids and potassium chloride in aqueous solutions at 298.15 K. J Therm Anal Calorim. 2011;104:789–93.
Nowicka B, Pałecz B, Belica S, H Piekarski H. The interactions between some N-acetyl-N′-methyl-L-α–amino acid amides and urea in water at 298.15 K. Thermochim Acta. 2006;448:41–3.
Palecz B, Belica S, Piekarski H, Jóźwiak A. Studies of homogeneous interactions of N-acetyl-N′-methyl-L-α-amino acid amides in water at 298.15 K. Thermochim Acta. 2009;489:1–4.
Piekarski H, Nowicka B. Calorimetric studies of interactions of some peptides with electrolytes, urea and ethanol in water at 298.15 K. J Therm Anal Calorim. 2010;102:31–6.
Friedman HL, Krishnan CV. Studies of hydrophobic bonding in aqueous alcohols: enthalpy measurements and model calculations. J Solution Chem. 1973;2:119–40.
Franks F, Padley M, Ried DS. Solute interactions in dilute aqueous solutions. Part 1—microcalorimetric study of the hydrophobic interaction. J Chem Soc Faraday Trans. 1. 1976;72:359–67.
McMillan WG, Mayer JE. The statistical thermodynamics of multicomponent systems. J Chem Phys. 1945;13:276–305.
Weissberg A, Proskauer ES, Riddick JA, Toops EE. Organic solvents. New York: Interscience Publishers; 1955.
Palecz B. The enthalpies of interaction of glycine with some amides and ureas in water at 25°C. J Solution Chem. 1995;24:537–50.
Lamanna R, Cannistraro S. Effect of ethanol addition upon the structure and the cooperativity of the water H bond network. Chem Phys. 1996;213:95–110.
Ludwig R. MNR relaxation studies in water-alcohol mixtures: the water-rich region. Chem Phys. 1995;195:329–37.
Desnoyers JE, Perron G, Avedikian L, Morel J-P. Enthalpies of the urea-tert-butanol-water system at 25°C. J Solution Chem. 1976;5:631–44.
Palecz B. Thermodynamics of interactions between zwitterions of several L-α-amino acids and ethanol in aqueous solution. Thermochim Acta. 2005;435:99–101.
SYu Noskov, Lamoureux G, Roux B. Molecular dynamics study of hydration in ethanol-water mixtures using a polarizable force field. J Phys Chem B. 2005;109:6705–13.
Palecz B, Nadolna A. Heterogeneous interaction between zwitterions of some L-α-amino acids and ethanol molecule in water at 298.15 K. Fluid Phase Equilib. 2006;250:49–52.
Palecz B. The enthalpies of interactions of some L-α-amino acids with urea molecule in aqueous solutions at 298.15 K. Amino Acids. 2004;27:299–303.
Eisenberg D, McLachlan AD. Solvation energy in protein folding and binding. Nature. 1986;319:199–203.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Pałecz, B., Smok, A. Study of the interaction between ethanol and natural amino acids containing ionic side groups in water at T = 298.15 K. J Therm Anal Calorim 111, 917–921 (2013). https://doi.org/10.1007/s10973-012-2278-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2278-6