Abstract
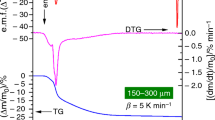
Zirconium hydroxide gel has been prepared by a novel aqueous gelation process by the controlled hydrolysis of zirconium oxychloride in the presence of sodium acetate. The gel thus formed has been subjected to thermal analysis: TG, DTG, and DSC. Thermal analysis shows that the gel is continuously dehydrated in the temperature range between room temperature and 500 °C. The total mass loss relative to the initial mass is about 44.1%. Thermal analysis shows that the decomposition takes place in three stages. The gel contains absorbed and coordinated water. In the second stage of dehydration, dehydration of the Zr(OH)4 gel also takes place along with the removal of the coordinated water. The DSC analysis coupled with TG and structural information, indicate that the exothermic processes between 349 and 460 °C can be attributed to the nucleation process of the formation of tetragonal zirconia, with phase transformation at 460 °C.






Similar content being viewed by others
References
Aramendia MA, Borau V, Jimenez C, Marinas JM, Porras A, Urbano FJ. Synthesis and characterization of ZrO2 as an acid-base catalyst: dehydration-dehydrogenation of propan-2-ol. J Chem Soc Faraday Trans. 1997;93:1431.
Kaspar J, Fornasiero P, Graziani M. Use of CeO2-based oxides in the three-way catalysis. Catal Today. 1999;50:285.
Shayachmetov U, Dranca I. Use of methods of thermal analysis in studying ceramic materials on the basis of Al2O3, ZrO2, Si3N4 SiC and inorganic binder. J Therm Anal Calorim. 2001;64:1153–61.
Gao P, Meng LJ, dos Santos MP, Teixeira V, Andritschky M. Study of ZrO2–Y2O3 films prepared by magnetron reactive sputtering. Thin Solid Films. 2000;32:377.
Xinlong Wang, Lianghu Wu, Jin Li J. Synergistic flame retarded poly (methyl methacrylate) by nano-ZrO2 and triphenylphosphate. J Therm Anal Calorim. 2011;103:741–6.
Davis H. Effect of pH on crystal phase of ZrO2 precipitated from solution and calcined at 600 °C. J Am Ceram Soc. 1996;67:168.
Nair AS, Renjis TTom, Suryanarayanan V, Pradeep T. ZrO2 bubbles from core shell nanoparticles. J Mater Chem. 2003;13:97.
Somiya S, Akiba T. Hydrothermal zirconia powders: a bibliography. J Eur Ceram Soc. 1999;19:81.
Picquart M, López T, Gómez R, Torres E, Moreno A, Garcia J. Dehydration and crystallization process in sol–gel zirconia: thermal and spectroscopic study. J Therm Anal Calorim. 2004;76:755.
Santos V, Zeni M, Bergmann CP, Hohemberger JM. Correlation between thermal treatment and tetragonal/monoclinic nanostructured zirconia powder obtained by sol–gel process. Adv Mater Sci. 2008;17:62.
Garrido Pedrosa AM, Souza MJB, Lima SH, Dulce MAMelo, Souza AG, Araujo AS. Influence of the synthesis methods of the DTG-TPR profiles of Pt/WOx-ZrO2 bifunctional catalysts. J Therm Anal Calorim. 2007;87:703–7.
Colon G, Aviles MA, Navio JA, Sanchez-Soto PJ. Thermal behaviour of TiO2-ZrO2 microcomposite prepared by chemical coating. J Therm Anal Calorim. 2002;67:229–38.
Jakubus P, Adamski A, Kurzawa M, Sojka Z. Texture of Zirconia obtained by forced hydrolysis of ZrOCl2 solutions influence of aging on thermal behaviour. J Thermal Anal Calorim. 2003;72:299–310.
Sato Taichi. Thermal decomposition of zirconium oxyhydroxide. J Therm Anal Calorim. 2005;69:255.
Strizhak PE, Tripol’skii AI, Gurnik TN, Tuzikov FV, Moroz ÉM, Konstantinova TE, Tuzikova NA, Kol’ko VP, Danilenko IA, Gorban OA. Effect of temperature on the structural characteristics of zirconium dioxide nanoparticles produced under conditions of microwave treatment. Theor Exp Chem. 2008;44:144.
Rendtorff NM, Suarez G, Sakka Y, Aglietti EF. Thermal behaviour of mullite-Zirconia–Zircon composites influence of Zirconia phase transformation. J Thermal Anal Calorim. 2011;104:569–76.
Hao Zhang, Yulong LU, Zhu Ke, Siu Guigu, Xiong Yonghong, Cao shui Xiong. Infrared spectra of nano metre granular Zirconia. J Phys Condens Matter. 1999;11:2035.
Ali A. HT-XRD, IR, Raman characterization studies of metastable phases emerging in the thermal genesis course of monoclinic zirconia via amorphous zirconium hydroxide: impacts of sulfate and phosphate additives. Thermochim Acta. 2002;387:29.
Palaniswami Manivasakan, Venkadachalam Rajendran, PremaRenjan Ranta. Synthesis of monoclinic and cubic ZrO2 nanoparticles from zircon. J Am Ceram Soc. 2011;94:1410.
Jayakumar S, Ananthapadmanabhan PV, Perumal K, Thiyagarajan TK, Mishra SC, Su LT, Tok AIY, Guo J. Characterization of nano-crystalline ZrO2 synthesized via reactive plasma processing. Mater Sci Eng. 2011;176:894.
Sharikov FYu, Almjasheva OV, Gusarov VV. Thermal analysis of formation of ZrO2 nanoparticles under hydrothermal conditions. Rus J Inorg Chem. 2006;51:1538.
Tahir Ahamed, Othman Mamat. The development and characterization of zirconia-silica sand nanoparticles composites. World J Nanosci Eng. 2001;1:7.
PDF # 501089 and PDF # 371484.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
George, A., Seena, P.T. Thermal studies on Zirconium hydroxide gel formed by aqueous gelation. J Therm Anal Calorim 110, 1037–1041 (2012). https://doi.org/10.1007/s10973-011-2042-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2042-3