Abstract
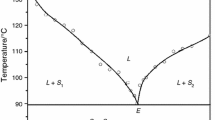
The solid–liquid phase equilibrium data of two binary organic systems, namely, urea (U)–3-aminophenol (AP) and 3-hydroxybenzaldehyde (HB)–β-napthaol (BN) show formation of a eutectic in each case. The enthalpies of fusion of the pure components and binary eutectics have been determined using differential scanning calorimeter (Mettler DSC-4000) system. The thermal properties of the materials such as heat of mixing, entropy of fusion, roughness parameter, interfacial energy and excess thermodynamic functions were computed using the enthalpy of fusion values. The microstructures of eutectics were developed using unidirectional thermal gradient and interested region were photographed.



Similar content being viewed by others
References
Rice JW, Suuberg EM. Thermodynamic study of (anthracene + aenzo[a]pyrene) solid mixtures. J Chem Thermodyn. 2010;42:1356–60.
Teng J, Liu S, Trivedi R. Growth and morphology of rod eutectics. Acta Mater. 2008;56:2819–33.
Sturz L, Witusiewicz VT, Hecht U, Rex S. Organic alloy systems suitable for the investigation of regular binary and ternary eutectic growth. J Cryst Growth. 2004;270:273–82.
Suk MJ, Leonartz K. Halo growth during unidirectional solidification of camphor-Naphthalene eutectic system. J Cryst Growth. 2000;213:141–9.
Teng J, Liu S. Re-determination of succinonitrile (SCN)-camphor phase diagram. J Cryst Growth. 2006;290:248–57.
Gunter P. Nonlinear optical effects and materials. Berlin: Springer-Verlag; 2000. p. 540.
Rajasekaran M, Anbusrinivasan P, Mojumdar SC. Growth, spectral and thermal characterization of 8-hydroxyquinoline. J Therm Anal Calorim. 2010;100:827–30.
Singh NB, Henningsen T, Hopkins RH, Mazelsky R, Hamacher RD, Supertzi EP, Hopkins FK, Zelmon DE, Singh OP. Nonlinear optical characteristics of binary organic system. J Cryst Growth. 1993;128:976–80.
Dwivedi Y, Kant S, Rai RN, Rai SB. Efficient white light generation from 2,3-diphenyl-1,2-dihydro-quinoxaline complex. Appl Phys B. 2010;101:639–42.
Carenco A, Jerphagnon J, Perigand A. Nonlinear optical properties of some m-disubstituted benzene derivatives. J Chem Phys. 1997;66:3806–13.
Rai RN, Ramasamy P, Lan CW. Synthesis crystal growth of binary organic NLO material UNBA. J Cryst Growth. 2002;235:499–504.
Rai RN, Mudunuri SR, Reddi RSB, Satuluri VSAK, Ganeshmoorthy S, Gupta PK. Crystal growth and nonlinear optical studies of m-dinitrobenzene doped urea. J Cryst Growth. 2011;321:72–7.
Prasad LG, Krishnakumar V, Shanmugam G, Nagalakshmi R. Structural, thermal and optical studies on 2-naphthol crystals. Cryst Res Technol. 2010;45:1057–63.
Paixao JA, Matos Beja A, Ramos Silva M, Alte da Veiga L, Serra AC. 3-Hydroxybenzaldehyde. Acta Cryst. 2000;C56:1348–50.
Dean JA. Lange’s handbook of chemistry. New York: McGraw-Hill; 1985.
Dwivedi Y, Kant S, Rai SB, Rai RN. Synthesis, physicochemical and optical characterization of novel fluorescing complex: o-phenylenediamine–benzoin. J Fluoresc. doi:10.1007/s10895-010-0808-9.
Rai US, Rai RN. Physical chemistry of organic eutectic and monotectic: hexamethylbenzene-succinonitrile system. Chem Mater. 1999;11(11):3031–6.
Reddi RSB, Satuluri VSAK, Rai US, Rai RN. Thermal, physicochemical and microstructural studies of binary organic eutectic systems. J Therm Anal Calorim. doi:10.1007/s10973-011-1478-9.
Rai RN. Phase diagram, optical, nonlinear optical, and physicochemical studies of the organic monotectic system: pentachloropyridine-succinonotrile. J Mater Res. 2004;19(5):1348–55.
Rai RN, Rai US. Solid–liquid equilibrium and thermochemical properties of organic eutectic in a monotectic system. Thermochim Acta. 2000;363:23–8.
Rai US, Rai RN. Physical chemistry of the organic analog of metal-metal eutectic and monotectic alloys. J Cryst Growth. 1998;191:234–42.
Rai US, Rai RN. Physical chemistry of organic eutectics. J Therm Anal Calorim. 1998;53:883–93.
Singh N, Singh NB, Rai US, Singh OP. Structure of eutectic melts: binding organic systems. Thermochim Acta. 1985;95:291–3.
Christian JW. The theory of phase transformation in metals and alloys. Oxford: Pergamon Press; 1965. p. 992.
Hunt JD, Jackson KA. Binary eutectic solidification. Trans Met Soc AIME. 1966;236:843–52.
Acknowledgements
Authors are grateful thanks to the Board of Research in Nuclear Science, for financial support, and also thanks to Department of Chemistry, BHU for providing infrastructure.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddi, R.S.B., Kumar Satuluri, V.S.A. & Rai, R.N. Solid–liquid equilibrium, thermal and physicochemical studies of organic eutectics. J Therm Anal Calorim 107, 183–188 (2012). https://doi.org/10.1007/s10973-011-1634-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1634-2