Abstract
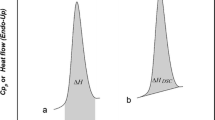
This article focuses on the development of an innovative method, based on thermodynamic considerations and with the use of Differential Scanning Calorimetry (DSC), for the estimation of the melting enthalpy of crystalline compounds which are metastable near their melting temperature. The curves obtained, at various heating rates, are analysed in two steps. In the first step, the area of a zone generated by the melting endothermic peak is calculated following a specific method. In the second step, the melting enthalpy is extracted from this area through an enthalpy balance. This method is applied to both identified crystallographic forms, named form I and form II, respectively, of Etiracetam (UCB Pharma). The results show that the melting enthalpy of the stable form II compare well with the ones obtained using conventional methods. The curves of the metastable form I present thermal instabilities (partial solid–solid polymorphic transition and beta-recrystallization) near the form I melting peak leading to difficulties for a direct determination of the melting enthalpy by conventional methods. The proposed method is therefore very useful for the estimation of the form I melting enthalpy.











Similar content being viewed by others
Notes
DSC figures are given in degree celsius. The program used does not allow the conversion to Kelvin.
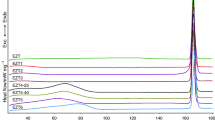
All initial and final temperature combinations, for each heating rate, are summarised in the Supporting information (SI, Tables S2, S3, S4 and S5).
These results are given in the Supporting information (SI, Figs. S5 and S6).
All initial and final temperatures combination, for each of the heating rates, are summarised in the Supporting information (SI, Tables S6, S7 and S8).
A zoom of each sub-figure of Fig. 11 is given in the Supporting information (SI, Fig. S7).
References
Mangin D, Puel F, Veesler S. Polymorphism in processes of crystallization in solution—a practical review. Org Process Res Dev. 2009;13:1241–53.
Veesler S, Ferte N, Costes MS, Czjzek S, Astier JP. Temperature and pH effect on the polymorphism of Aprotinin (PBTI) in sodium bromide solutions. J Cryst Growth Des. 2004;4(6):1137–41.
Giron D. Thermal analysis and calorimetric methods in the characterisation of polymorphs and solvates. Thermochim Acta. 1995;248:1–59.
Boettinger WJ, Kattner UR, Moon KW, Perepezko JH. DTA and heat-flow DSC measurements of alloy melting and freezing, NIST Recommended Practice Guide. 2006.
Kolbe E, Wilson LA, Hartel R. A round robin evaluation of differential scanning calorimetry to measure transition enthalpy and temperatures. J Food Eng. 1999;40:95–9.
Riesen R. Thermal Analysis Information for Users METTLER TOLEDO: Choosing the right baseline, User Com 25. 2007.
Wunderlich B. Thermal analysis. New York: Academic Press; 1990. p. 277–80.
Greco A, Maffezzoli A. Correction of melting peaks of different PE grades accounting for heat transfer in DSC samples. Polym Test. 2008;27(1):61–74.
Herman, C; PhD Thesis, Université Libre de Bruxelles, Belgium. 2010. Contribution à l'étude de la cristallisation, par refroidissement en cuve agitée, de substances d'intérêt pharmaceutique présentant un polymorphisme cristallin. http://theses.ulb.ac.be/ETD-db/collection/available/ULBetd-12082009-155716/ (2010).
Brown ME. Handbook of thermal analysis and calorimetry. Amsterdam: Elsevier; 1998.
Marini A, Bernenni V, Flor G, Massarotti V, Riccardi R. An analysis of the factor affecting the peak shape and the quantitative reliability of a heat flux DSC cell. Thermochim Acta. 1985;95(2):419–24.
Marini A, Berbenni V, Massarotti V, Flor G. On the quantitative reliability on heat flux DSC. J Therm Anal Calorim. 1988;33(1):337–42.
Grooff D, Villiers MM, Liebenberg W. Thermal methods for evaluating polymorphic transitions in Nefedipine. Thermochim Acta. 2007;454:33–42.
Lara-Ochoa F, Pérez GE, Mijangos-Santiago F. Calorimetric determinations and theoretical calculations of polymorphs of thalidomide. J Mol Struct. 2007;840(1–3):97–106.
Herman C, Leyssens T, Vermylen V, Halloin V, Haut B. Towards an accurate and precise determination of the solid–solid transition temperature of enantiotropic systems. J Chem Thermodyn. 2011;43:677–82.
Herman C, Haut B, Halloin V, Vermylen V, Leyssens T. Towards the determination of the solubilities of the two enantiotropically-related crystallographic forms of Etiracetam in methanol. Org Process Res Dev. 2011 (accepted).
Burger A, Ramberger R. On the polymorphism of pharmaceutical and other molecular crystals-I. Theory of thermodynamics rules. Mikrochim Acta. 1979;2:259–71.
Drebushchak VA, Drebushchak TN, Chukanov NV, Boldyreva EV. Transitions among five polymorphs of chlorpropamide near the melting point. J Therm Anal Calorim. 2008;93(2):343–51.
Acknowledgements
Christelle Herman acknowledges technical support from UCB Pharma, based in Braine l’Alleud (Brussels), Belgium, and financial support from the Fonds National de la Recherche Scientifique (F.R.S.-F.N.R.S.), Belgium.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Herman, C., Leyssens, T., Vermylen, V. et al. A new approach for the estimation of the melting enthalpy of metastable crystalline compounds using differential scanning calorimetry. J Therm Anal Calorim 107, 777–788 (2012). https://doi.org/10.1007/s10973-011-1555-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1555-0