Abstract
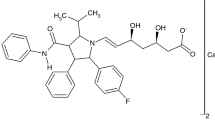
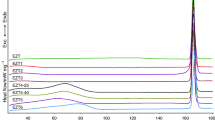
Thermogravimetry (TG) and differential scanning calorimetry (DSC) are useful techniques that have been successfully applied in the pharmaceutical industry to reveal important information regarding the physicochemical properties of drug and excipient molecules such as polymorphism, stability, purity, and formulation compatibility among others. In this study, lovastatin was studied by TG, DSC, and other techniques such as Fourier transform infrared spectroscopy, optical microscopy, X-ray diffraction, chromatography, and mass spectrometry. Lovastatin showed melting point at 445 K and thermal stability up to 535 K. It presented morphological polymorphism, which in the drug has the same unit cell, but with different crystal habits. Preservative excipient butylhydroxyanisole (BHA) causes amorphization of lovastatin crystallites and, therefore is incompatible with lovastatin. Degradation by hydrolysis was observed under neutral, acid, and basic conditions. The active degradation product, lovastatin hydroxyacid, was obtained after neutral and basic hydrolysis.








Similar content being viewed by others
References
Craig DQM, Reading M. Thermal analysis of pharmaceuticals. New York: CRC Press; 2007.
Wendlandt WW. Thermal analysis. 3rd ed. New York: Wiley; 1986.
Gabbott P. Principles and applications of thermal analysis. Oxford: Blackwell Publishing; 2008.
Caira MR, Foppoli A, Sangalli ME, Zema L, Giordano F. Thermal and structural properties of ambroxol polymorphs. J Therm Anal Calorim. 2004;77:653–62.
Freitas MN, Alves R, Matos JR, Marchetti JM. Thermal analysis applied in the osmotic tablets pre-formulation studies. J Therm Anal Calorim. 2007;87:905–11.
Hassan MA, Salem MS, Sueliman MS, Najib NM. Characterization of famotidine polymorphic forms. Int J Pharm. 1997;149:227–32.
Laszcz M, Kosmacinska B, Korczak K, Smigielska B, Glice M, Maruszak W, Groman A, Beczkowicz H, Zelazko L. Study on compatibility of imatinib mesylate with pharmaceutical excipients. J Therm Anal Calorim. 2007;88:305–10.
Leitão MLP, Canotilho J, Cruz MSC, Pereira JC, Souza AT, Redinha JS. Study of polymorphism from DSC melting curves: polymorphs of terfenadine. J Therm Anal Calorim. 2002;68:397–412.
Macedo RO, Nascimento TG, Veras JWE. Compatibility and stability studies of propranolol hydrochloride binary mixtures and tablets for TG and DSC-Photovisual. J Therm Anal Calorim. 2002;67:483–9.
Oliveira GGG, Ferraz HG, Matos JSR. Thermoanalytical study of glibenclamide and excipients. J Therm Anal Calorim. 2005;79:267–70.
Porter WW III, Elie SC, Matzger AJ. Polymorphism in carbamazepina cocrystals. Cryst Growth Des. 2008;8:14–6.
Stulzer HK, Rodrigues PO, Cardoso TM, Matos JSR, Silva MAS. Compatibility studies between captopril and pharmaceutical excipients used in tablets formulations. J Therm Anal Calorim. 2008;91:323–8.
Hebert PR, Gaziano JM, Chan KS, Hennekens CH. Cholesterol lowering with statins drugs, risk of stroke and total mortality. An overview of randomized trial. JAMA. 1997;278:313–21.
Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Semes R. Cholesterol treatment trialists (CTT) collaborators: efficacy and safety of cholesterol-lowering treatment: propective meta-analysis of data from 90,056 participants in 14 randomized trials of statins. Lancet. 2005;366:1267–78.
Ward S, Lloyd JM, Pandor A, Holmes M, Ara R, Ryan A, Yeo W, Payne N. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess. 2007;11:1–160.
Scientific Steering Committee on behalf of the Simon Broome Register Group. Mortality in treated heterozygous familial hypercholesterolaemia: implications for clinical management. Atherosclerosis. 1999;142:105–12.
Rodenburg J, Vissers MN, Wiegman A, Trotsenburg ASP, Graaf A, Groot E, Wijburg FA, Kastelein JJP, Hutten BA. Statin treatment in children with familial hypercholesterolemia: the younger, the better. Circulation. 2007;116:664–8.
The Merck Index, Version 12:3 [Cd-Rom], Chapman & Hall, New York; 1999.
Moffat AC, Osselton MD, Widdop B. Clarke’s analysis of drugs and poisons: in pharmaceuticals, body fluids and postmortem material, vol. 2. 3rd ed. London: Pharmaceutical Press; 2004.
Amidon GL, Kasim NA, Whitehouse M, Ramachandran C, Bermejo M, Lennernas H, Hussain AS, Junginger HE, Stavchansky SA, Midha KK, Shah VP. Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Mol Pharm. 2004;1:85–96.
FDA—Food and Drug Administration. Guidance for industry. Dissolution testing of immediate release solid oral dosage forms. FDA, CDER, 1997.
Balestrieri F, Magri AD, Magri AL, Marini D, Sacchini A. Application of differencial scanning calorimetry to the study of drug-excipient compatibility. Thermochim Acta. 1996;285:337–45.
Bazzo GC, Segatto Silva GC. Estudo termoanalítico de comprimidos revestidos contendo captopril através de termogravimetria (TG) e calorimetria exploratória diferencial (DSC). Braz J Pharm Sci. 2005;41:315–22.
Cides LCS, Araújo AAS, Santos-Filho M, Matos JR. Thermal behaviour, compatibility study and decomposition kinetics of glimepiride under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2006;84:441–5.
Carini JP, Pavei C, Silva APC, Machado G, Mexias AS, Pereira VP, Fialho SL, Mayorga P. Solid state evaluation of some thalidomide raw materials. Int J Pharm. 2009;372:17–23.
Sperandeo NR, Karlsson A, Cuffini S, Pagola S, Stephens PW. The crystal structure and physicochemical characteristics of 2-hydroxy-N-[3(5)-pyrazolyl]-1,4-naphthoquinone-4-imine, a new antitrypanosomal compound. AAPS Pharm. Sci Technol. 2005;6:E655–63.
Tedesco E, Giron D, Pfeffer S. Crystal structure elucidation and morphology study of pharmaceuticals in development. Cryst Eng Commun. 2002;4:393–400.
The United States pharmacopeia. 31st ed. Rockville: United States Pharmacopeial Convention; 2008.
ICH Q2 (R1)—International conference on harmonization of technical requirements for the registration of drugs for human use. Validation of analytical procedures: text and methodology Q2(R1), Nov, 2005. http://www.ich.org/LOB/media/MEDIA417.pdf.
Snyder LR, Kirkland JJ, Glajch JL. Pratical HPLC method development. 2nd ed. New York: Wiley; 1997.
British pharmacopoeia 2007, Version 11.0 [Cd-Rom], London: The Stationery Office; 2007.
ICH Q1A (R2)—International conference on harmonization of technical requirements for the registration of drugs for human use. Stability testing of new drug substances and products Q1A(R2), Feb, 2003. http://www.ich.org/LOB/media/MEDIA419.pdf.
Sundaram V, Kharkar MR, Yarraguntla SR, Gudipati S, Mandava VNBR. Amorphous simvastatin. US patent application publication, US 2006/0223882 A1, Oct. 5; 2006.
Macedo RO, Nascimento TG, Aragão CFS, Barreto Gomes AP. Application of thermal analysis in the characterization of anti-hypertensive drugs. J Therm Anal Calorim. 2000;59:657–61.
Ferguson HF, Frurip DJ, Pastor AJ, Peerey LM, Whiting LF. A review of analytical applications of calorimetry. Therm Acta. 2000;363:1–21.
Rodrigues PO, Cardoso TFM, Silva MAS, Matos JR. Aplicação de técnicas termoanalíticas na caracterização, determinação da pureza e cinética de degradação da zidovudina (AZT). Acta Farm Bonaer. 2005;24:383–7.
Lueje AA, Pastine J, Squella JA, Nunez-Vergara LJ. Assessment of the hydrolytic degradation of lovastatin by HPLC. J Chil Chem Soc. 2005;50:639–46.
Moffat AC, Osselton MD, Widdop B. Clarke’s analysis of drugs and poisons: in pharmaceuticals, body fluids and postmortem material, vol. 1. 3rd ed. London: Pharmaceutical Press; 2004.
Acknowledgements
FAPES, CNPq, and FAPEMIG are acknowledged for financial support and Laboratório Teuto Brasileiro for the raw materials donation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshida, M.I., Oliveira, M.A., Gomes, E.C.L. et al. Thermal characterization of lovastatin in pharmaceutical formulations. J Therm Anal Calorim 106, 657–664 (2011). https://doi.org/10.1007/s10973-011-1510-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1510-0