Abstract
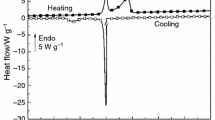
The self-aggregation of the ionic tetrapeptide RWDW (R = arginine, W = tryptophan, D = aspartic acid) was studied at three temperatures (15, 25 and 35 °C) by different experimental techniques such as atomic force microscopy (AFM), isothermal titration calorimetry (ITC) and differential scanning calorimetry (DSC). AFM was used to investigate the morphology of the aggregates; the AFM images at 15 °C showed the presence of a dense network of entangled fibres, while at 35 °C the peptide assembled into sparse globular and fibrillar structures. Moreover, the calorimetric experiments showed that in all cases the disaggregation process is endothermic and dependent on the investigated temperature. Both the enthalpy of disaggregation and the cac change with temperature. In particular, at 35 °C, we obtained the lower enthalpy of disaggregation and higher cac, showing that the disaggregation process is favoured at high temperature. The DSC scans strengthen the hypothesis that the RWDW aggregation is a rather complex phenomenon.




Similar content being viewed by others
References
Zhang S. Emerging biological materials through molecular selfassembly. Biotechnol Adv. 2002;20:321–39.
Reches M, Gazit E. Rigid, self-assembled hydrogel composed of a modified aromatic dipeptide. Curr Nanosci. 2006;2:105–11.
Lomakin A, Teplow DB, Kirschner DA, Benedek GB. Kinetic theory of fibrillogenesis of amyloid β–protein. Proc Natl Acad Sci USA. 1997;94:7942–7.
Dobson CM. Protein folding and its links with human disease. Biochem Soc Symp. 2001;68:1–26.
Yu L, Ding J. Injectable hydrogels as unique biomedical materials. Chem Soc Rev. 2008;37:1473–81.
Holmes TC, de Lacalle S, Su X, Liu G, Rich A, Zhang S. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci USA. 2000;97:6728–33.
Deming TJ. Synthetic polypeptides for biomedical applications. Prog Polym Sci. 2007;32:858–75.
Hosseinkhani H, Hosseinkhani M, Kobayashi H. Design of tissue-engineered nanoscaffold through self-assembly of peptide amphiphile. J Bioact Compat Polym. 2006;21:277–96.
Zhang S, Holmes TC, Lockshin C, Rich A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc Natl Acad Sci USA. 1993;90:3334–8.
Desii A, Chiellini F, Duce C, Ghezzi L, Monti S, Solaro R, Tiné MR. Influence of structural features on the self-assembly of short ionic oligopeptide. J Polym Sci Part A: Polym Chem. 2010;48:889–97.
Desii A, Duce C, Ghezzi L, Monti S, Solaro R, Tiné MR. Investigation of the self-assembly of hydrophobic self-complementary ionic tetrapeptides. J Therm Anal Cal. 2009;97:789–90.
Desii A, Chiellini F, Di Stefano R, Tiné MR, Solaro R. Hydrogel scaffolds by self-Assembly of a complementary ionic tetrapeptide. J Polym Sci Part A: Polym Chem. 2010;48:986–90.
Pochan DJ, Schneider JP, Kretsinger J, Ozbas B, Rajagopal K, Haines L. Thermally reversible hydrogels via intramolecular folding and consequent self-assembly of a de novo designed peptide. J Am Chem Soc. 2003;125(39):11802–3.
Jeong B, Bae YH, Lee DS, Kim SW. Biodegradable block copolymers as injectable drug-delivery systems. Nature. 1997;388:860–2.
Privalov PL. Stability of protein-structure and hydrophobic interactions. Biol Chem Hoppe Seyler. 1988;369:199.
Ye Z, Zhang H, Luo H, Wang S, Zhou Q, DU X, Tang C, Chen L, Liu J, Shi YK, Zhang EY, Ellis-Behnke R, Zhao X. Temperature and pH effects on biophysical and morphological properties of self-assembling peptide RADA16-I. J Pept Sci. 2008;14:152–62.
Ramachandran S, Taraban MB, Trewhella J, Gryczynski I, Gryczynski Z, Yu YB. Effect of temperature during assembly on the structure and mechanical properties of peptide-based materials. Biomacromolecules. 2010;11:1502–6.
Garidel P, Hildebrand A. Thermodynamic properties of association colloids. J Therm Anal Cal. 2005;82:483–9.
Majhi RP, Blume A. Thermodynamic characterization of temperature-induced micellization and demicellization of detergents studied by differential scanning calorimetry. Langmuir. 2001;17:3844–51.
Kljin JE, Kevelam J, Engberts JBFN. Aggregation behavior of mono-endcapped hydrophobically modified poly(sodium acrylate)s in aqueous solution. J Coll Interf Sci. 2000;226:76–82.
Acknowledgements
The authors are grateful to the reviewer for helpful suggestions. The financial support by MIUR, INSTM and Fondazione Cassa di Risparmio di Pisa is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiné, M.R., Alderighi, M., Duce, C. et al. Effect of temperature on self-assembly of an ionic tetrapeptide. J Therm Anal Calorim 103, 75–80 (2011). https://doi.org/10.1007/s10973-010-1060-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1060-x