Abstract
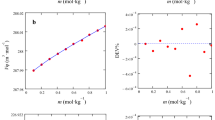
In this work we report the experimental measurements of excess molar enthalpy and excess molar volume, at 298.15 K and atmospheric pressure, on ethylammonium nitrate (EAN) and propylammonium nitrate (PAN) + water mixtures. Positive enthalpies were found for the two systems (maximum, at x 1 around 0.37 correspond to about 700 and 900 J mol−1 for EAN and PAN respectively). As the hydrophobic/hydrophilic ratio increases, along with the length of the alkyl chain in the ionic liquids, ILs, the specific interactions IL-water become less important. The excess molar volumes, V E, are negative over the entire composition range for the two binary mixtures. They have similar values but curves exhibit a different asymmetric shape and around equimolar composition they intersect each other. This behaviour: positive H E and negative V E, is not very common.



Similar content being viewed by others
References
Welton T. Chem Rev. 1998;99:2071–84.
Rogers RD, Seddon KR. Science. 2003;302:792–3.
Li RX. Green solvents: synthesis and applications of ionic liquids. Beijing: Chemistry Technology Press; 2004.
Kandil ME, Marsh KN, Goodwin ARH. J Chem Eng Data. 2007;52:2382–7.
Diedrichs A, Gmehling J. Fluid Phase Equilib. 2006;244:68–77.
Gomez E, Gonzales B, Calvar N, Tojo E, Dominguez A. J Chem Eng Data. 2006;51:2096–102.
Guan W, Li L, Wang H, Tong J, Yang JZ. J Therm Anal Cal. 2008;94:507–10.
Garcia-Miaja G, Troncoso J, Romanì L. J Chem Thermodyn. 2009;41:161–6.
Rodriguez H, Brennecke JF. J Chem Eng Data. 2006;51:2145–55.
Allen M, Evans DF, Lumry R. J Solut Chem. 1985;14:549–60.
Bou Malham I, Letellier P, Mayaffre A, Turmine M. J Chem Thermodyn. 2007;39:1132–43.
Hadded M, Biquard M, Letellier P, Shaal R. Can J Chem. 1985;63:565–70.
Weiser ME. Pure Appl Chem. 2006;78:2051–66.
Greaves TL, Drummond CJ. Chem Rev. 2008;108:206–37.
Riddick JA, Bunger WB, Sakano TK. Organic solvents, physical properties and methods of purification. New York: Wiley; 1986.
Monk P, Wadsö I. Acta Chem Scan. 1968;22:1842–52.
Marsh KN. Int Data Ser Sel Data Mix Ser A 1973;1–5.
Marsh KN, Richards AE. Aust J Chem. 1980;33:212132.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is a tribute to Dr. Henry V. Kehiaian from Prof. Bruno Marongiu and coworkers.
Rights and permissions
About this article
Cite this article
Porcedda, S., Marongiu, B., Schirru, M. et al. Excess enthalpy and excess volume for binary systems of two ionic liquids + water. J Therm Anal Calorim 103, 29–33 (2011). https://doi.org/10.1007/s10973-010-1000-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1000-9