Abstract
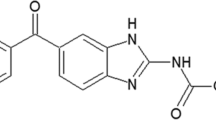
Primaquine (PQ) is the drug of choice for the radical cure of Plasmodium vivax malaria, and currently being administered in solid dosage form. In this study, the compatibility studies were carried out using differential scanning calorimetry (DSC), thermogravimetry (TG), and fourier transformed infrared (FT-IR). Non-isothermal and isothermal methods were employed to investigate kinetic parameters under nitrogen and air atmospheres using TG. The DSC investigations obtained by physical mixtures showed slight alterations in the melting temperatures of PQ with some excipients. The FT-IR confirmed the possible interactions obtained by DSC for the physical mixtures with PQ and lactose, magnesium stearate and mannitol. The results showed that the thermal decomposition followed a zero order kinetic in both atmospheres in non-isothermal method. The activation energy in both methods using nitrogen atmosphere was similar, and in air atmosphere the activation energy decreased.














Similar content being viewed by others
References
Baird JK, Fryauff DJ, Hoffman SL. Primaquine for prevention of malaria in travelers. Clin Infect Dis. 2003;37:1659–67.
Vale N, Moreira R, Gomes P. Primaquine revisited six decades after its discovery. Eur J Med Chem. 2009;44:937–53.
Bhadra A, Yadav K, Bhadra S, Jain NK. Glycodendrimeric nanoparticulate carriers of primaquine phosphate for liver targeting. Int J Pharm. 2005;295:221–33.
Gaspar R, Prat V, Roland M. Nanoparticles of polyisohexylcyanoacrylate (PIHCA) as carriers of primaquine: formulation, physico-chemical characterization and acute toxicity. Int J Pharm. 1991;68:111–9.
Green MD, D’Souza MJ, Holbrook JM, Wirtz RA. In vitro and in vivo evaluation of albumin-encapsulated primaquine diphosphate prepared by nebulization into heated oil. J Microencapsul. 2004;21:433–44.
Mayorga P, Puisieux F, Couarraze G. Formulation study of a transdermal delivery system of primaquine. Int J Pharm. 1996;132:71–9.
Jackson K, Young D, Pant S. Drug-excipient interaction and their affect on absorption. Res Focus. 2000;3:336–45.
Mura P, Faucci MT, Manderioli A, Bramanti G, Ceccarelli L. Compatibility study between ibuproxam and pharmaceutical excipients using differential scanning calorimetry, hot-stage microscopy and scanning electron microscopy. J Pharm Biomed Anal. 1998;18:151–63.
Cides LCS, Araújo AAS, Santos-Filho M, Matos JR. Thermal behavior, compatibility study and decomposition kinetics of glimepiride under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2006;84:441–5.
Nunes RS, Semaan FS, Riga AT, Cavalheiro ETG. Thermal behavior of verapamil hydrocholide and its association with excipients. J Therm Anal Calorim; 2009. doi:10.1007/s10973-009-0072-x.
Giron D. Contribution of thermal methods and related techniques to the rational development of pharmaceuticals, Part 1. PSTT. 1998;1:191–9.
Giron D. Contribution of thermal methods and related techniques to the rational development of pharmaceuticals, Part 2. PSTT. 1998;1:262–8.
Silva MAS, Kelmann RG, Foppa T, Cruz AP, Bertol CD, Sartori T, et al. Thermoanalytical study of fluoxetine hydrochloride. J Therm Anal Calorim. 2007;87:463–7.
Stulzer HK, Rodrigues PO, Cardoso TM, Matos JSR, Silva MAS. Compatibility studies between captopril and pharmaceutical excipients used in tablets formulations. J Therm Anal Calorim. 2008;91:323–8.
Sashina ES, Janowska G, Zaborski M, Vnuchkin AV. Compatibility of fibroin/chitosan and fibroin/cellulose blends studied by thermal analysis. J Therm Anal Calorim. 2007;89:887–91.
Gennaro AR. Remington’s the pharmaceutical sciences and practice of pharmacy. Philadelphia: Lippincott, Williams & Wilkins; 2004.
Mura P, Gratteri P, Faucci MT. Compatibility studies of multicomponent tablet formulations DSC and experimental mixture design. J Therm Anal Calorim. 2002;68:541–51.
Kiss D, Zelkó R, Novak CS, Éhen ZS. Application of DSC and NIRS to study the compatibility of metronidazole with different pharmaceutical excipients. J Therm Anal Calorim. 2006;84:447–51.
Bruni G, Amici L, Berbenni V, Marini A, Orlandi A. Drug-excipient compatibility studies: search of interaction indicators. J Therm Anal Calorim. 2002;68:561–73.
Felix FS, Cides LCS, Angnes L, Matos JR. Thermal behavior study and decomposition kinetics of salbutamol under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2009;95:877–80.
Burnham L, Dollimore D, Alexander K. Kinetic study of the drug acetazolamide using thermogravimetry. Thermochim Acta. 2002;392:127–33.
Ozawa T. Thermal analysis—review and prospect. Thermochim Acta. 2000;355:35–42.
Al-Badr AA. Primaquine diphosphate: comprehensive profile. Profiles Drug Subst Excipients Relat Methodol. 2005;32:153–207.
British Pharmacopoeia. 3ed. London; 1999.
Verma RK, Garg S. Selection of excipients for extended release formulations of glipizide through drug-excipient compatibility testing. J Pharm Biomed Anal. 2005;38:633–44.
Oliveira GGG, Ferraz HFG, Matos JSR. Thermoanalytical study of glibenclamide and excipients. J Therm Anal Calorim. 2005;79:267–70.
Lerdkanchanaporn S, Dollimore D, Alexander KS. A thermogravimetric study of ascorbic acid and its excipients in pharmaceutical formulations. Thermochimica Acta. 1996;284:115–26.
Bugay DE. Characterization of the solid-state: 2. Spectroscopic techniques. Adv Drug Deliv Rev. 2001;48:43–65.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bertol, C.D., Cruz, A.P., Stulzer, H.K. et al. Thermal decomposition kinetics and compatibility studies of primaquine under isothermal and non-isothermal conditions. J Therm Anal Calorim 102, 187–192 (2010). https://doi.org/10.1007/s10973-009-0540-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0540-3