Abstract
Isothermal titration calorimetry has been used to determine the stoichiometry, formation constants and thermodynamic parameters (ΔG o, ΔH, ΔS) for the formation of the citrate complexes with the Mn2+, Co2+, Ni2+ and Zn2+ ions. The measurements were run in Cacodylate, Pipes and Mes buffer solutions with a pH of 6, at 298.15 K. A constant ionic strength of 100 mM was maintained with NaClO4. The influence of a metal ion on its interaction energy with the citrate ions and the stability of the resulting complexes have been discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ions of the citric acid (2-hydroxy-1,2,3-propanetricarboxylic acid; H4Cit) occur in small amounts in the majority of living organisms to act as bioactive ligands implicated in a number of biochemical processes [1–5]. Owing to their capacity to form thermodynamically stable complexes with a variety of metal ions, they found widespread use in food and pharmaceutical industries as well as in medicine. Over the past few years much attention has been paid to the synthesis of nanomaterials using metal citrates as precursors [6–9].
Thermodynamic stability of complexes is crucial for processes occurring in living organisms, as it determines, amongst others, biological and pharmacological activities of complex compounds and plays an important role in the safety of their application. It is important to realize that the knowledge of thermodynamic parameters of reactions enables a better understanding of the processes involving complex compounds than that of simple equilibrium constants [10, 11]. Thermodynamic characteristics of a reaction also enable determination of the relationship between the structure of a ligand and chemical properties of new compounds, thus contributing to optimization of the conditions for their synthesis [12]. For this reason it seemed worthwhile to determine thermodynamic characteristics for the reactions of the citrate ions with some transition metal ions.
Experimental
Materials
All reagents: C6H5O7Na3·2H2O (sodium citrate dihydrate), Mn(NO3)2·6H2O, Co(NO3)2·6H2O, Ni(NO3)2·6H2O, Zn(NO3)2·6H2O, NaClO4, Cacodylate (Cacodylic acid sodium salt trihydrate), Pipes (1,4-Piperazinediethanesulfonic acid) and Mes 2-(N-Morpholino)ethanesulfonic acid) were purchased from Aldrich Chemical Corp. These compounds were used without further purification.
Isothermal titration calorimetry (ITC)
All the ITC experiments were run at 298.15 K using an AutoITC isothermal titration calorimeter (MicroCal Inc., Northampton, USA) with a 1.4491-mL sample and a reference cell. The reference cell was filled with distilled water. The data, specifically the heat normalized per mole of injectant, were processed with Origin 7 from MicroCal. An initial 2-μL injection sample was discarded from each data set to remove the effect of titrant diffusion across the syringe tip during the equilibration process. The experiment consisted of injection (29 injections, 2 μL for the first injection only) of a ca. 10–15-mM solution of appropriate salt into the reaction cell initially containing buffered solution of a ca. 1 mM sodium citrate (ionic strength I = 100 mM NaClO4). A background titration was performed using identical titrant with the buffer solution placed in the sample cell. The result was subtracted from each experimental titration to account for the heat of dilution. All the solutions were degassed before titrations were performed. Titrant was injected at 5-min intervals to ensure that the titration peak returned to the baseline prior to the next injection. Each injection lasted 20 s. To achieve a homogeneous mixing in the cell, the stirrer speed was kept constant at 300 rpm. Calibration of the AutoITC calorimeter was carried out using electrically generated heat pulses. The CaCl2–EDTA titration was performed to check the apparatus and the results (n, K, ΔH) were compared with those obtained for the same samples (test kit) at MicroCal.
Results and discussion
Thermodynamic parameters of interaction of the citrate ion with the Mn2+, Co2+, Ni2+ and Zn2+ ions, determined by the ITC technique in the Cacodylate, Pipes, and Mes buffer solutions witha pH of 6, at 298.15 K, are summarized in Table 1. The equilibrium constants, binding enthalpies and reaction stoichiometries were obtained from ITC experiments by fitting binding isotherms, using nonlinear least-squares procedures, to a model that assumes a single set of identical binding sites. From the above experimental parameters, the free energy of binding (ΔG o) and entropy change (ΔS) could be determined from the standard thermodynamic relationship, ΔG o = −RT ln K obs = Δobs H − TΔS.
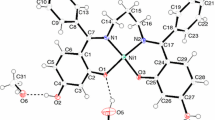
The stoichiometry of the compounds indicates that at a pH of 6 almost equimolar metal/ligand complexes are formed. X-ray crystallographic results have shown that the citrate ions act as tridentate ligands [13–15]. Oxygen atoms of two carboxylic groups and an oxygen atom of the hydroxyl group participate in the metal binding (Fig. 1). A carboxyl group at the central carbon atom, C(3), is almost perpendicular to the carbon backbone, C(1)–C(2)–C(3)–C(4)–C(5), and is situated on one plane with the hydroxyl group. A third donor is the oxygen atom of the terminal carboxyl group, C(1) [16, 17]. A similar type of metal binding can also be expected in solutions.
In solution with a pH of 6, the citrate ions occur mostly as the H2Cit2− and HCit3− species, the equilibrium being displaced largely towards the former ones [18] ([H2Cit2−]:[HCit−] equals approximately 4:1). In general, the stability constant (conditional stability constant) of considered reactions can be defined as
where Me2+ denotes Mn2+, Co2+, Ni2+ or Zn2+ ion.
Logarithmic stability constants of the examined metal citrates [MeHCit]− are comparable with the values of the \( \log K({\text{Me}}^{{ 2 + }} + {\text{HCit}}^{3 - } \rightleftarrows {\text{MeHCit}}^{ - } ) \): 3.54–3.67, 4.16–4.83, 4.99–5.11 and 4.25–4.9 for [MnHCit]−, [CoHCit]−, [NiHCit]− and [ZnHCit]−, respectively, as found in the literature [19].
The fairly strong protonation of the carboxyl group not involved in the metal binding seems to impede the formation of binuclear species of the type [Co2(C6H5O7)2(H2O)4]2− [20] and [Ni2(C6H5O7)2(H2O)4]2− [21].
The overall energetic effect of the complexation consists mainly of the dehydration of the metal cation and the ligand, Δdehyd H > 0, on the one hand, and the formation of new ion–ligand bonds, Δbind H < 0, on the other hand.
In the set of the compounds studied, the energy released by metal ion–citrate interaction is overcompensated by endothermic dehydration of the ion (Δdehyd H > Δbind H). Positive values of the measured reaction enthalpy, ΔH obs, show the thermodynamic stability of the complexes to be strongly dependent on the entropy change and to increase in the order Mn2+ < Co2+ = Zn2+ < Ni2+. An additional factor affecting experimental Δobs H values is the identity of buffer solution. That is, the enthalpy change decreases with an increase in buffer ionization energy. The energy is the sum of all energetic effects accompanying the reaction, i.e. the enthalpy due to the heat of complex formation, Δbind H, which is independent of the identity of buffer, and the energy due to proton transfer from the ligand to the buffer [22–24]:
where Δion H buf is the enthalpy of buffer ionization, and Δn is the number of protons exchanged during binding.
For a given pH value, the relationship between Δobs H and Δion H buf is a straight line whose slope corresponds to the number of protons interchanged during the reaction. The ionization energies of the buffers used in this study are −2.97, 11.21 and 14.81 kJ mol−1 for Cacodylate, Pipes and Mes, respectively [25]. The number of protons interchanged during citrate complex formation, determined in this way at a pH of 6, are 0.2(±0.12), 0.27(±0.03), 0.27(±0.02) and 0.19(±0.03) for Mn2+, Co2+, Ni2+ and Zn2+, respectively (Fig. 2). The Δobs H versus Δion H buf relationship is a decreasing function (Δn < 0), this indicating that in the complexation process the proton is transferred from the ligand onto a buffer component [26].
Arithmetic means of the enthalpy changes for interaction of the ions with the citrate ligand (Δbind H), accounting for the number of protons exchanged during complex formation and the ionization enthalpy of the Cacodylate, Pipes and Mes components, are 9.2, 8.83, 6.32 and 5.56 kJ mol−1 for Mn2+/HCit3−, Co2+/HCit3−, Ni2+/HCit3− and Zn2+/HCit3−, respectively. The determined thermodynamic characteristics of the complexes show that the entropy term, TΔS, has a greater impact on stability of the resulting species than does the enthalpy term ΔH associated with the energy of the donor–acceptor bonds.
Conclusions
Interaction of the metal ions with the citrate ligand in solution of a pH of 6, at 298.15 K, is an endothermic process resulting in the formation of 1:1 complexes. The enthalpy change of the reaction, Δbind H, accounting for the energy contribution due to binding proton by a buffer component, has been found to depend on the metal ion identity and to decrease in the order Mn2+ > Co2+ > Ni2+ > Zn2+. Thermodynamic stability of the complexes is determined by the entropy term that overcompensates the positive value of the enthalpy change. These findings may be useful for optimization of synthetic procedures of the complexes. They would also enable to predict the influence of the presence of metal ions on biological activity of citrate ions.
References
Martin RB. Citrate binding of Al3+ and Fe3+. J Inorg Biochem. 1989;28:181–7.
Beinert H, Kennedy MC. Engineering of protein bound iron-sulfur clusters. A tool for the study of protein and cluster chemistry and mechanism of iron-sulfur enzymes. Eur J Biochem. 1989;186:5–15.
Brynhildsen L, Allard B. Influence of metal complexation on the metabolism of citrate by Klebsiella oxytoca. Biometals. 1994;7:163–9.
Krom BP, Warner JB, Konings WN, Lolkema JS. Complementary metal ion specificity of the metal-citrate transporters CitM and CitH of Bacillus subtilis. J Bacteriol. 2000;182:6374–81.
Lippard SJ. Principles of bioinorganic chemistry. Mill Valley, CA: University Science Books; 1994. p. 352.
Bi J, Wu L, Li Z, Wang X, Fu X. A citrate complex process to prepare nanocrystalline PbBi2Nb2O9 at a low temperature. Mater Lett. 2008;62:155–8.
Mesquita A, Bernardi MIB, Maia LJQ, Mastelaro VR. Synthesis and characterization of Pb1−x La x TiO3 nanocrystalline powders. J Therm Anal Calorim. 2007;87:747–51.
Delmon B. Preparation of heterogeneous catalysts. J Therm Anal Calorim. 2007;90:49–65.
Fuentes RO, Baker RT. Synthesis of nanocrystalline CeO2–ZrO2 solid solutions by a citrate complexation route: a thermochemical and structural study. J Phys Chem C. 2009;113:914–24.
Velazquez-Campoy A, Luque I, Freire E. The application of thermodynamic methods in drug design. Thermochim Acta. 2001;380:217–27.
Freire E. Isothermal titration calorimetry: controlling binding forces in lead optimization. Drug Discov Today Technol. 2004;1:295–9.
Holdgate GA, Ward WHJ. Measurements of binding thermodynamics in drug discovery. Drug Discov Today. 2005;10:1543–50.
Deng Y-F, Zhou Z-H, Wan H-L, Ng SW. Δ-Aqua-S-citrato(2-)manganese(II). Acta Crystallogr. 2003;E59:m310–2.
Zhou Z-H, Deng Y-F, Wan H-L. Structural diversities of cobalt(II) coordination polymers with citric acid. Cryst Growth Des. 2005;5:1109–17.
Zhang G, Yang G, Ma JS. Versatile framework solids constructed from divalent transition metals and citric acid: syntheses, crystal structures, and thermal behaviors. Cryst Growth Des. 2006;6:375–81.
Glusker JP. Citrate conformation and chelation: enzymatic implications. Acc Chem Res. 1980;13:345–52.
Carrell HL. Metal chelation versus internal hydrogen bonding of the α-hydroxy carboxylate group. J Am Chem Soc. 1987;109:8067–71.
Al-Khaldi MH, Nasr-El-Din HA, Mehta S, Al-Aamri AD. Reaction of citric acid with calcite. Chem Eng Sci. 2007;62:5880–96.
Sillen LG, Martel AE. Stability constants of metal–ion complexes. Spec. Publ. 17. London, Great Britain: The Chemical Society; 1966.
Kotsakis N, Raptopoulou CP, Tangoulis V, Terzis A, Giapintzakis J, Jakusch T, et al. Correlations of synthetic, spectroscopic, structural, and speciation studies in the biologically relevant cobalt(II)-citrate system: the tale of the first aqueous dinuclear cobalt(II)-citrate complex. Inorg Chem. 2003;42:22–31.
Baker EN, Baker HM, Anderson BF, Reeves RD. Chelation of nickel(II) by citrate. The crystal structure of a nickel–citrate complex, K2[Ni(C6H5O7)(H2O)2]2·4H2O. Inorg Chim Acta. 1983;78:281–5.
Baker BM, Murphy KP. Evaluation of linked protonation effects in protein binding reactions using Isothermal Titration Calorimetry. Biophys J. 1996;71:2049–55.
Fukada H, Takahashi K. Enthalpy and heat capacity changes for the proton dissociation of various buffer components in 0.1 M potassium chloride. Proteins. 1998;33:159–66.
Haq I, O’Brien R, Lagunavicius A, Siksnys V, Ladbury JE. Specific DNA recognition by the type II restriction endonuclease MunI: the effect of pH. Biochemistry. 2001;40:14960–7.
Goldberg RN, Kishore N, Lennen RM. Thermodynamic quantities for the ionization reactions of buffers. J Phys Chem Ref Data. 2002;31:231–70.
Gomez J, Freire E. Thermodynamic mapping of the inhibitor site if the aspartic protease endothiapepsin. J Mol Biol. 1995;252:337–50.
Acknowledgements
This research was supported by the Polish State Committee for Scientific Research under grant DS/8230-4-0088-9.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Wyrzykowski, D., Chmurzyński, L. Thermodynamics of citrate complexation with Mn2+, Co2+, Ni2+ and Zn2+ ions. J Therm Anal Calorim 102, 61–64 (2010). https://doi.org/10.1007/s10973-009-0523-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0523-4