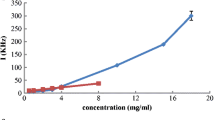
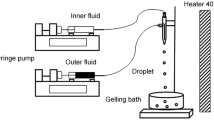
Abstract
Alginate has been established as a very versatile material in the preparation of hydrogel capsules for trapping therapeutic biomolecules and cells. The physico-chemical properties, the mechanism and the processing of gel formation are now well established. In the frame of a project aiming at the exploitation of encapsulation of therapeutic proteins in alginate gel particles, the procedure of preparation, characterization, gel-drying and re-hydrating has been explored for the shelf-life of the encapsulated biomolecules. Here, the results of a calorimetric study on the freezing and dehydration process of alginate micro-capsules is presented. The work aims at the description of water state(s) and its removal under “controlled conditions” in the presence of bioprotectant sugars.






Similar content being viewed by others
References
Haug A, Smidsrod O. Selectivity of some anionic polymers for divalent metal ions. Acta Chem Scand. 1970;24:843–54.
Kohn R. Ion binding on polyuronates—alginate and pectin. Pure Appl Chem. 1975;42:371–97.
Donati I, Holtan S, Mørch YA, Borgogna M, Dentini M, Skjåk-Braek G. New hypothesis on the role of alternating sequences in calcium-alginate gels. Biomacromolecules. 2005;6:1031–40.
Rees DA. Polysaccharide shapes—outline studies in biology. London: Chapman and Hall; 1977. p. 1–110.
Painter TJ. In: Aspinall GO, editor. The polysaccharides, vol. 2. New York: Academic Press; 1983. p. 196–285.
Donati I, Benegas JC, Cesàro A, Paoletti S. Specific interactions versus counterion condensation, 2: theoretical treatment within the counterion condensation theory. Biomacromolecules. 2006;7:1587–96.
Cesàro A, Delben F, Paoletti S. Interaction of divalent cations with polyuronates. J Chem Soc, Faraday Trans. 1988;84:2573–84.
Hatakeyama H, Hatakeyama T. Interaction between water and hydrophilic polymers. Thermochim Acta. 1998;308:3–22.
Takahashi M, Hatakeyama T, Hatakeyama H. Phenomenological theory describing the behaviour of non-freezing water in structure formation process of polysaccharide aqueous solutions. Carbohydr Polym. 2000;41:91–5.
Monaselidze J, Kiladze M, Tananashvili D, Barbakadze S, Naskidashvili A, Khizanishvili A, et al. Free and bound water influence on Spirulina platensis serviva. J Therm Anal Calorim. 2006;84:613–18.
Champagne CP, Fustier P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr Opin Biotechnol. 2007;18:184–90.
Ubbink J, Krüger J. Physical approaches for the delivery of active ingredients in foods. Trends Food Sci Technol. 2006;17:244–54.
Manojlovic V, Rajic N, Djonlagic J, Obradovic B, Nedovic V, Bugarski B. Application of electrostatic extrusion—flavour encapsulation and controlled release. Sensors. 2008;8:1488–96.
Kaushik JK, Bath R. Why is trehalose an exceptional protein stabilizer? J Biol Chem. 2003;278:26458–65.
Cesàro A, De Giacomo O, Sussich F. Water interplay in trehalose polymorphism. Food Chem. 2008;106:1318–28.
Borgogna M, Bellich B, Zorzin L, Lapasin R, Cesàro A. Food microencapsulation of bioactive compounds: rheological and thermal characterisation of nonconventional gelling system. Food Chem. 2009. doi:10.1016/j.foodchem.2009.07.043.
Gombotz WR, Pettit DK. Biodegradable polymers for protein and peptide drug delivery. Bioconjug Chem. 1995;6:332–51.
Reis CP, Neufeld RJ, Vilela S, Ribeiro AJ, Veiga F. Review and current status of emulsion/dispersion technology using an internal gelation process for the design of alginate particles. J Microencapsul. 2006;23:245–57.
Gombotz WR, Wee SF. Protein release from alginate matrices. Adv Drug Deliv Rev. 1998;31:267–85.
Chen L, Remondetto GE, Subirade M. Food protein-based materials as nutraceutical delivery systems. Trends Food Sci Technol. 2006;17:272–83.
Martins S, Sarmento B, Souto EB, Ferreira DC. Insulin-loaded alginate microspheres for oral delivery—effect of polysaccharide reinforcement on physicochemical properties and release profile. Carbohydr Polym. 2007;69:725–31.
Chandramouli V, Kailasapathy K, Peiris P, Jones M. An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. J Microbiol Methods. 2004;56:27–35.
Mutalik V, Manjeshwar LS, Wali A, Sairam M, Raju KVSN, Aminabhavi TM. Thermodynamics/hydrodynamics of aqueous polymer solutions and dynamic mechanical characterization of solid films of chitosan, sodium alginate, guar gum, hydroxy ethyl cellulose and hydroxypropyl methylcellulose at different temperatures. Carbohydr Polym. 2006;65:9–21.
Faroongsarng D, Sukonrat P. Thermal behavior of water in the selected starch- and cellulose-based polymeric hydrogels. Int J Pharm. 2008;352:152–8.
Hay JN, Laity PR. Observations of water migration during thermoporometry studies of cellulose films. Polymer. 2000;41:6171–80.
Thu B, Skjåk-Bræk G, Micali F, Vittur F, Rizzo F. The spatial distribution of calcium in alginate gel beads analysed by synchrotronradiation induced X-ray emission (SRIXE). Carbohydr Res. 1997;297:101–5.
Rault J, Gref R, Ping ZH, Nguyen QT, Neel J. Glass transition temperature regulation effect in a poly(vinyl alcohol)-water system. Polymer. 1995;36:1655–61.
Nakamura K, Nishimura Y, Hatakeyama T, Hatakeyama H. Thermal properties of water insoluble alginate films containing di- and trivalent cations. Thermochim Acta. 1995;267:343–53.
Buitink J, Leprince O. Glass formation in plant anhydrobiotes: survival in the dry state. Cryobiology. 2004;48:215–28.
Sussich F. Trehalose polymorphism and its role in anhydrobiosis. Ph.D. Thesis, University of Trieste; 2004.
Sussich F, Bortoluzzi S, Cesàro A. Trehalose dehydration under confined conditions. Thermochim Acta. 2002;391:137–50.
Sussich F, Cesàro A. Transitions and phenomenology of α,α-trehalose polymorphs inter-conversion. J Therm Anal Calorim. 2000;62:757–68.
Ping H, Nguyen QT, Chen SM, Zhou JQ, Ding YD. States of water in different hydrophilic polymers—DSC and FTIR studies. Polymer. 2001;42:8461–7.
Lewicki PP. Water as the determinant of food engineering properties. A review. J Food Eng. 2004;61:483–95.
Acknowledgements
The present results have been achieved in part within the EU Project FP6 NanoBioPharmaceutics (NMP 026723-2). M.B. is recipient of a University Grant from University of Trieste (Progetto Giovani Ricercatori 2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Barbara Bellich and Massimiliano Borgogna are contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Bellich, B., Borgogna, M., Carnio, D. et al. Thermal behavior of water in micro-particles based on alginate gel. J Therm Anal Calorim 97, 871–878 (2009). https://doi.org/10.1007/s10973-009-0392-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0392-x