Abstract
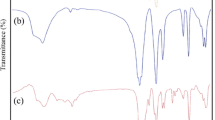
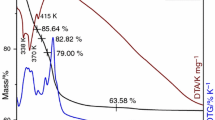
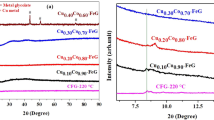
This paper presents a study for the preparation of CoxFe3−xO4 (x = 0.02, 0.2, 0.5, 0.8, 1.0, 1.1, 1.5) nanoparticles, starting from metal nitrates: Co(NO3)2·6H2O, Fe(NO3)3·9H2O and ethylene glycol (C2H6O2). By heating the solutions metal nitrates-ethylene glycol, the redox reaction took place between the anion NO3 − and OH–(CH2)2–OH with formation of carboxylate anions. The resulted carboxylate anions reacted with Co(II) and Fe(III) cations to form coordinative compounds which are precursors for cobalt ferrite. XRD and magnetic measurements have evidenced the formation of cobalt ferrite for all studied molar ratios. The average diameter of the cobalt ferrite crystallites was estimated from XRD data and showed values in the range 10–20 nm. The crystallites size depends on the annealing temperature. The magnetization of the synthesized samples depends on the molar ratio Co/Fe and on the annealing temperature.









Similar content being viewed by others
References
Kubota M, Kanazawa Y, Nasu K, Moritake K, Kawaji H, Atake T, Ichiyanagi Y. Effect of heat treatment on magnetic MgFe2O4 nanoparticles. J Therm Anal Calorim. 2008;98:461–63.
Mendelovici E, Villalba R, Sagarzazu A. Distinctive cobalt ferrites prepared by the thermal-transformation alkoxide route. Thermochim Acta. 1998;318:51–6.
Franco Jr A, Lima ECO, Novak MA, Wells PR. Synthesis of nanoparticles of CoxFe(3−x)O4 by combustion reaction method. J Magn Magn Mater. 2007;308:198–202.
Liu C, Rondione AJ, Zhang ZJ. Synthesis of magnetic spinel ferrite CoFe2O4 nanoparticles from ferric salt and characterization of the size-dependent superparamagnetic properties. Pure Appl Chem. 2000;72:37–45.
Franco Jr A, Zapf V. Temperature dependence of magnetic anisotropy in nanoparticles of CoxFe(3−x)O4. J Magn Magn Matter. 2008;320:709–13.
Li X, Kutal C. Synthesis and characterization of superpara-magnetic CoxFe3−xO4 nanoparticles. J Alloys Comp. 2003;349:264–8.
Cote LJ, Teja AS, Wilkinson AP, Zhang ZJ. Continuous hydrothermal synthesis of CoFe2O4 nanoparticles. Fluid Phase Equilibria. 2003;210:307–17.
Chirtop E, Mirtov I, Ion RM, Iliescu M. A low temperature path to the preparation of CoFe2O4 ferrite. J Optoel Adv Mater. 2000;2(4):379–84.
Horng L, Chern G, Chen MC, Kang PC, Lee DS. Magnetic anisotropic properties in Fe3O4 and CoFe2O4 ferrite epitaxy thin films. J Magn Magn Mater. 2004;270:389–96.
Dippong T, Stoia M, Stefanescu M. Study on the obtaining of CoxOy starting from Co(NO3)2·6H2O and ethylene glycol. Proceeding of XIVth, symposium on analytical and environmental problems, 24 September 2007, Szegen, Hungary, p. 134–8.
Stefanescu M, Stefanescu O, Stoia M, Lazau C. Thermal decomposition of some metal-organic precursors. Fe2O3 nanoparticles. J Therm Anal Cal. 2007;88:27–32.
Stefanescu M, Dippong T, Stoia M, Stefanescu O. Study on the obtaining of cobalt oxides by thermal decomposition of some complex combinations, undispersed and dispersed in SiO2 matrix. J Therm Anal Calorim. 2008;94:389–93.
Prasad R, Sulaxna, Kumar A. Kinetics of thermal decomposition of iron(III) dicarboxylate complexes. J Therm Anal Calorim. 2005;81:441–50.
Acknowledgements
This work was supported by the National Project No. 71-026 NANOPART and financed by CNMP-ANCS through Romanian Ministry of Education and Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stefanescu, M., Stoia, M., Caizer, C. et al. Preparation of CoxFe3−xO4 nanoparticles by thermal decomposition of some organo-metallic precursors. J Therm Anal Calorim 97, 245 (2009). https://doi.org/10.1007/s10973-009-0250-x
Published:
DOI: https://doi.org/10.1007/s10973-009-0250-x