Abstract
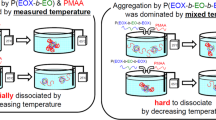
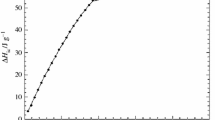
The water + cyclodextrin + poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) mixtures have been investigated to explore the temperature effect on the aggregation of the copolymer in the presence of cyclodextrins (CDs). The CDs with different cavity sizes were chosen because they may include either the hydrophilic poly(ethylene oxides) block or both kinds of blocks. The differential scanning calorimetry and viscosity experiments straightforwardly evidenced that the critical micellar temperature is shifted to larger values by adding a CD which is able to include the middle poly(propylene oxide) block while it is not influenced by the presence of CD which is selective to the poly(ethylene oxide) block. The enthalpy of aggregation decreases upon the CD addition for all the investigated systems.







Similar content being viewed by others
References
Del Valle EMM. Cyclodextrins and their uses: a review. Process Biochem. 2004;39:1033–46.
De Lisi R, Lazzara G, Milioto S, Muratore N, Terekhova IV. Heat capacity study to evidence the interactions between cyclodextrin and surfactant in the monomeric and micellized states. Langmuir. 2003;19:7188–95.
De Lisi R, Lazzara G, Milioto S, Muratore N. Volumes and heat capacities of the aqueous sodium dodecanoate/sodium perfluorooctanoate mixtures in the presence of β-cyclodextrins. Phys Chem Chem Phys. 2003;5:5084–90.
Bernat V, Ringdard-Lefebvre C, Le Bas G, Perly B, Djedaïni-Pilard F. Inclusion complex of n-octyl β-d-glucopyranoside and α-cyclodextrin in aqueous solutions: thermodynamic and structural characterization. Langmuir. 2008;24:3140–9.
Haller J, Katze U. Complexation versus micelle formation: α-cyclodextrin + n-decyltrimethylammonium bromide aqueous solutions. Chem Phys Lett. 2008;463:94–8.
Guerrero-Martínez A, González-Gaitano G, Viñas MH, Tardajos G. Inclusion complexes between β-cyclodextrin and a gemini surfactant in aqueous solution: an NMR study. J Phys Chem B. 2006;110:13819–28.
Mehta SK, Bhasin KK, Shilpee D, Singla ML. Micellar behavior of aqueous solutions of dodecyldimethylethylammonium bromide, dodecyltrimethylammonium chloride and tetradecyltrimethylammonium chloride in the presence of α-, β-, HPβ- and γ-cyclodextrins. J Colloid Interface Sci. 2008;321:442–51.
Nicolle GM, Merbach AE. Destruction of perfluoroalkyl surfactant aggregates by β-cyclodextrin. Chem Commun. 2004;7:854−5.
Terekhova IV, De Lisi R, Lazzara G, Milioto S, Muratore N. Volume and heat capacity studies to evidence interactions between cyclodextrins and nicotinic acid in water. J Therm Anal Calorim. 2008;92:285–90.
Wan Yunus WMZ, Taylor J, Bloor DM, Hall DG, Wyn-Jones E. Electrochemical measurements on the binding of sodium dodecyl sulfate and dodecyltrimethylammonium bromide with α- and β-cyclodextrins. J Phys Chem. 1992;96:8979–82.
Funasaki N, Ishikawa S, Neya S. Proton NMR study of α-cyclodextrin inclusion of short-chain surfactants. J Phys Chem B. 2003;107:10094−9.
Guo QX, Li ZZ, Ren T, Zhu XQ, Liu YC. Inclusion complexation of sodium alkyl sulfates with β-cyclodextrin. A 1H NMR study. J Inclusion Phenom Mol Recognit Chem. 1994;17:149–56.
Cabaleiro-Lago C, Nilsson M, Soderman O. Self-diffusion NMR studies of the host−guest interaction between β-cyclodextrin and alkyltrimethylammonium bromide surfactants. Langmuir. 2005;21:11637–44.
Xing H, Lin S, Yan P, Jin-Xin X. Demicellization of a mixture of cationic−anionic hydrogenated/fluorinated surfactants through selective inclusion by α- and β-cyclodextrin. Langmuir. 2008;24:10654–64.
Xing H, Lin SS, Yan P, Xiao JX, Chen YM. NMR studies on selectivity of β-cyclodextrin to fluorinated/hydrogenated surfactant mixtures. J Phys Chem B. 2007;111:8089–95.
Milioto S, Bakshi MS, Crisantino R, De Lisi R. Thermodynamic properties of water-β-cyclodextrin-dodecylsurfactant ternary systems. J Solution Chem. 1995;24:103–20.
De Lisi R, Milioto S, De Giacomo A, Inglese A. Thermodynamic properties of sodium n-perfluoroalkanoates in water and in water + cyclodextrins mixtures. Langmuir. 1999;15:5014–22.
De Lisi R, Milioto S, Pellerito A, Inglese A. Thermodynamic properties of sodium n-alkanecarboxylates in water and in water + cyclodextrins mixtures. Langmuir. 1998;14:6045–53.
Harada A, Li J, Kamachi M. Preparation and properties of inclusion complexes of polyethylene glycol with α-cyclodextrin. Macromolecules. 1993;26:5698–703.
Harada A, Kamachi M. Complex formation between poly(ethylene glycol) and α-cyclodextrin. Macromolecules. 1990;23:2821–3.
Harada A, Kamachi M. The molecular necklace: a rotaxane containing many threaded α-cyclodextrins. Nature. 1992;356:325–7.
Wenz G, Han BH, Muller A. Cyclodextrin rotaxanes and polyrotaxanes. Chem Rev. 2006;106:782–817.
Hunt MA, Tonelli AE, Balik CM. Effect of guest hydrophobicity on water sorption behavior of oligomer/α-cyclodextrin inclusion complexes. J Phys Chem B. 2007;111:3853–8.
Peet J, Rusa CC, Hunt MA, Tonelli AE, Balik CM. Solid-state complexation of poly(ethylene glycol) with α-cyclodextrin. Macromolecules. 2005;38:537–41.
Lo Nostro P, Lopes JR, Cardelli C. Formation of cyclodextrin-based polypseudorotaxanes: solvent effect and kinetic study. Langmuir. 2001;17:4610–5.
Jing B, Chen X, Hao J, Qiu H, Chai Y, Zhang G. Supramolecular self-assembly of polypseudorotaxanes in ionic liquid. Colloids Surf A Physicochem Eng Asp. 2007;192:51–5.
Lazzara G, Milioto S. Copolymer−cyclodextrin inclusion complexes in water and in the solid state. A physico-chemical study. J Phys Chem B. 2008;12:11887–95.
Li J, Ni X, Zhou Z, Leong KW. Preparation and characterization of polypseudorotaxanes based on block-selected inclusion complexation between poly(propylene oxide)-poly(ethylene oxide)-poly(propylene oxide) triblock copolymers and α-cyclodextrin. J Am Chem Soc. 2003;125:1788–95.
Gaitano GG, Brown W, Tardajos G. Inclusion complexes between cyclodextrins and triblock copolymers in aqueous solution: a dynamic and static light-scattering study. J Phys Chem B. 1997;101:710–9.
Li J, Li X, Zhou Z, Ni X, Leong KW. Formation of supramolecular hydrogels induced by inclusion complexation between pluronics and α-cyclodextrin. Macromolecules. 2001;34:7236–7.
Joseph J, Dreiss CA, Cosgrove T, Pedersen JS. Rupturing polymeric micelles with cyclodextrins. Langmuir. 2007;23:460–6.
Fujita H, Ooya T, Yui N. Synthesis and characterization of a polyrotaxane consisting of β-cyclodextrins and a poly(ethylene glycol)-poly(propylene glycol) triblock copolymer. Macromol Chem Phys. 1999;200:706–13.
Udachin KA, Wilson LD, Ripmeester JA. Solid polyrotaxanes of polyethylene glycol and cyclodextrins: the single crystal X-ray structure of PEG−β-cyclodextrin. J Am Chem Soc. 2000;122:12375–6.
Lazzara G, Milioto S, Muratore N. Solubilization of an organic solute in aqueous solutions of unimeric block copolymers and their mixtures with monomeric surfactant: volume, surface tension, differential scanning calorimetry, viscosity, and fluorescence spectroscopy studies. J Phys Chem B. 2008;112:5616–25.
Da Silva RC, Olofsson G, Schillen K, Loh W. Influence of ionic surfactants on the aggregation of poly(ethylene oxide)−poly(propylene oxide)−poly(ethylene oxide) block copolymers studied by differential scanning and isothermal titration calorimetry. J Phys Chem B. 2008;106:1239–46.
Dwyer C, Viebke C, Meadows J. Propofol induced micelle formation in aqueous block copolymer solutions. Colloid Surf A Physicochem Eng Asp. 2005;254:23–30.
De Lisi R, Lazzara G, Lombardo R, Milioto S, Muratore N, Turco Liveri ML. Thermodynamic behavior of non-ionic tri-block copolymers in water at three temperatures. J Solution Chem. 2006;35:659–78.
Alexandridis P, Holzwarth JF, Hatton TA. Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions: thermodynamics of copolymer association. Macromolecules. 1994;27:2414–25.
Patterson I, Armstrong J, Chowdhry B, Leharne S. Thermodynamic model fitting of the calorimetric output obtained for aqueous solutions of oxyethylene-oxypropylene-oxyethylene triblock copolymers. Langmuir. 1997;13:2219–26.
De Lisi R, Lazzara G, Milioto S, Muratore N. Volumes of aqueous block copolymers based on poly(propylene oxides) and poly(ethylene oxides) in a large temperature range: a quantitative description. J Chem Thermodyn. 2006;38:1344–50.
Lazzara G, Milioto S, Gradzielski M. The solubilisation behaviour of some dichloroalkanes in aqueous solutions of PEO-PPO-PEO triblock copolymers: a dynamic light scattering, fluorescence spectroscopy, and SANS study. Phys Chem Chem Phys. 2006;8:2299–312.
Wen XG, Verrall RE, Liu GJ. Effect of anesthetic molecules (halothane and isoflurane) on the aggregation behavior of POE-POP-POE triblock copolymers. J Phys Chem B. 1997;103:2620–6.
Ikeda T, Lee WK, Ooya T, Yui N. Thermodynamic analysis of inclusion complexation between α-cyclodextrin-based molecular tube and poly(ethylene oxide)-block-poly(tetrahydrofuran)-block-poly(ethylene oxide) triblock copolymer. J Phys Chem B. 2003;107:14–9.
Acknowledgements
The work was financially supported by the University of Palermo.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Lisi, R., Lazzara, G. Aggregation in aqueous media of tri-block copolymers tuned by the molecular selectivity of cyclodextrins. J Therm Anal Calorim 97, 797–803 (2009). https://doi.org/10.1007/s10973-009-0223-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0223-0