Summary
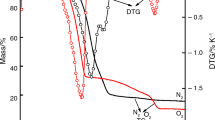
Samples of electrolytic manganese dioxide (EMD) were chemically reduced using 2-propanol under reflux (82°C) for 1, 2, 3, 6 and 24 h intervals. XRD analysis showed that the γ-MnO2 structure was preserved although the lattice dimensions were observed to increase with increasing degree of reduction to accommodate the intercalation of protons. The exception was the 24 h reduced sample which contained two phases; γ -MnO2 and γ -MnOOH. Three regions of decomposition in the range of 50 to 1000°C were observed using thermogravimetric analysis coupled with mass spectrometry (TG-MS) and were accounted for as water removal below 390°C, reduction of MnO2 to Mn2O3 between 400 and 600°C, and Mn2O3 to Mn3O4 between 600 and 1000°C. Again the exception proved to be the 24 h reduced sample which was observed to decompose predominantly in one step between 400 and 600°C directly to Mn3O4.
Similar content being viewed by others

Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liu, B., Thomas, P., Williams, R. et al. Thermal characterisation of chemically reduced electrolytic manganese dioxide. J Therm Anal Calorim 80, 625–629 (2005). https://doi.org/10.1007/s10973-005-0704-8
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-0704-8