Summary
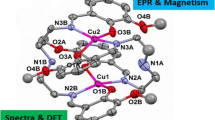
The reaction of CuCl with 2HNQ, (viz. 2-hydroxy-1,4-naphthoquinone), in methanol results in [Cu<Subscript>2</Subscript>(II,II)(4HNSQ)<Subscript>2</Subscript>(ONQ)<Subscript>2</Subscript>(H<Subscript>2</Subscript>O)<Subscript>4</Subscript>], Cu-3 complex; [where ONQ is the deprotonated oxidized form of ligand (viz. 2-oxido-1,4-naphthoquinone) and 4HNSQ one electron reduced tautomeric form of the ligand (i.e. 4-hydroxy-1,2-naphthosemiquinone)]. The mixed valent redox ligation is confirmed in [9] by us. In present report complex Cu-3 investigated by variable temperature magnetic susceptibility measurements (SQUID), X and Q-band EPR, DSC and CV techniques. A break is observed in the <Emphasis Type=”Italic”>x</Emphasis><Subscript> m</Subscript><Superscript>-1</Superscript> <Emphasis Type=”Italic”>vs</Emphasis>. <Emphasis Type=”Italic”>T</Emphasis> plot ~200 K in Cu-3 which is attributed to a phase transition. In Cu-3 a quintet state (<Emphasis Type=”Italic”>S=2</Emphasis>) is populated above 200 K by the molecular association of two exo Cu(II)(4HNSQ) units via hydrogen bonding between Cu(ONQ) unit of endo ligands in dimer. Magnetic susceptibility data is treated with tetramer model with <Emphasis Type=”Italic”>S</Emphasis>=1/2,1/2,1/2,1/2. The interdimer triplet-triplet interaction (<Emphasis Type=”Italic”>J</Emphasis>) in two [Cu(4HNSQ)] units and intradimer (z<Emphasis Type=”Italic”>J</Emphasis><Subscript>1</Subscript>) interaction between [Cu(II)(4HNSQ)] are best fitted with <Emphasis Type=”Italic”>J</Emphasis>= -50 cm<Superscript>-1</Superscript> and <Emphasis Type=”Italic”>zJ</Emphasis><Subscript>1</Subscript>=28 cm<Superscript>-1</Superscript>, respectively, using <Emphasis Type=”Italic”>g</Emphasis>=2.2. 'Quintet-triplet' phase transition occurs with an enthalpy change of 31.83 kJ mol<Superscript>-1</Superscript> estimated from DSC. Cu(II) ⇔Cu(I) and NSQ⇔CAT redox couples at <Emphasis Type=”Italic”>E</Emphasis><Subscript>1/2</Subscript>=0.68 V and <Emphasis Type=”Italic”>E</Emphasis><Subscript>1/2</Subscript> = -1.12 V, respectively are result of exo ligands and Cu(II) ions interaction, while shifts of ligand based peaks viz. NQ→NSQ and NSQ⇔CAT at -0.44 and -0.67 V towards positive potential on complexation are due to electron transfer interactions between endo ligand and Cu(II) ion.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bakare, P. Thermal, magnetic and spectral studies of metal-quinone complexes. J Therm Anal Calorim 79, 669–675 (2005). https://doi.org/10.1007/s10973-005-0594-9
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-0594-9