Abstract
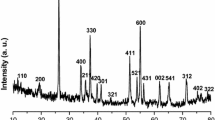
In present study, nanospheres of CeO2 are fabricated via utilizing a solvothermal mixed solvent technique at a low temperature. Using a three-electrode set up, the electrochemical activity of CeO2 nanospheres in 2.0 M alkaline medium was evaluated. At a scan range of 5 mV s−1, the material displayed large stability, the ability to work at high rates, and columbic efficiency, like specific capacitance value of 1435 F g−1 with specific energy of 87.89 Whkg−1 as well the power density of 1.0986 Wkg−1. The outstanding outcome of the CeO2 nanosphere is due to its mesoporous structure and high electrical double-layer capacitance of 6.35 mF. The nanospheres morphology of CeO2 was responsible for increased conductivity that allows ions to pass easily, and the improved findings show that the procedure employed to make the oxides, which is beneficial for future generations and may be used to produce a variety of oxides that will resolve the energy issues.
Graphical Abstract

Highlights
-
Controlled morphology of CeO2 nanoballs was fabricated with efficient and economical solvothermal method.
-
The capacitive properties of the CeO2 nanoball were determined with three-electrode system under 2 M KOH.
-
The electrochemical result of CeO2 displays a high specific capacitance value of 1435 F g−1, specific energy of 87.89 Wh kg−1, and specific power of 1.0986 Wkg−1.
-
The enhanced supercapacitive property of CeO2 was attributed to diverse morphology, larger surface area, and small crystallite size that permits faster ionic transport.



Similar content being viewed by others
References
Mendoza R, Oliva J, Padmasree KP, Oliva AI, Mtz-Enriquez AI, Zakhidov A (2022) Highly efficient textile supercapacitors made with face masks waste and thermoelectric Ca3Co4O9-δ oxide. J Energy Storage 46:103818.
Bakhoum DT, Oyedotun KO, Sarr S, Sylla NF, Maphiri VM, Ndiaye NM, Ngom BD, Manyala N (2022) A study of porous carbon structures derived from composite of cross-linked polymers and reduced graphene oxide for supercapacitor applications. J Energy Storage 51:104476.
Kumar R, Sahoo S, Tan WK, Kawamura G, Matsuda A, Kar KK (2021) Microwave-assisted thin reduced graphene oxide-cobalt oxide nanoparticles as hybrids for electrode materials in supercapacitor. J Energy Storage 40:102724.
Guo W, Lian X, Tian Y, Yang T, Wang S (2021) Facile fabrication 1D/2D/3D Co3O4 nanostructure in hydrothermal synthesis for enhanced supercapacitor performance. J Energy Storage 38:102586.
Kanaujiya N, Kumar N, Singh M, Sharma Y, Varma GD (2021) CoMn2O4 nanoparticles decorated on 2D MoS2 frame: a synergetic energy storage composite material for practical supercapacitor applications. J Energy Storage 35:102302.
Sarfraz M, Shakir I (2017) Recent advances in layered double hydroxides as electrode materials for high-performance electrochemical energy storage devices. J Energy Storage 13:103–122.
Choi HJ, Jung SM, Seo JM, Chang DW, Dai L, Baek JB (2012) Graphene for energy conversion and storage in fuel cells and supercapacitors. Nano Energy 1:534–551.
Zhu X (2022) Recent advances of transition metal oxides and chalcogenides in pseudo-capacitors and hybrid capacitors: a review of structures, synthetic strategies, and mechanism studies. J Energy Storage 49:104148.
Shehzad W, Karim MRA, Iqbal MZ, Shahzad N, Ali A (2022) Sono-chemical assisted synthesis of carbon nanotubes-nickel phosphate nanocomposites with excellent energy density and cyclic stability for supercapattery applications. J Energy Storage 54:105231.
Xiao J, Tong H, Jin F, Gong D, Chen X, Wu Y, Zhou Y, Shen L, Zhang X (2022) Heterostructure NiS2/NiCo2S4 nanosheets array on carbon nanotubes sponge electrode with high specific capacitance for supercapacitors. J Power Sources 518:230763.
Guo Y, Wei Y, Shu L, Li A, Zhang J, Wang R (2022) Structure-related RuSe2 nanoparticles and their application in supercapacitors. Colloids Surf A Physicochem Eng Asp 651:129702.
Lu Q, Omar A, Hantusch M, Oswald S, Ding L, Nielsch K, Mikhailova D (2022) Dendrite-free and corrosion-resistant sodium metal anode for enhanced sodium batteries. Appl Surf Sci 600:154168.
Pandey U, Singh AK, Sharma C (2022) Development of anti-corrosive novel nickel-graphene oxide-polypyrrole composite coatings on mild steel employing electrodeposition technique. Synth Met 290:117135.
Hassannejad H, Nouri A, Lajevardi SA, Molavi FK (2022) Novel approach for the synthesis of highly corrosion resistant and electrically conductive cerium hexaboride coating, J Mater Eng Perform 31:8906–8913.
Padmanathan N, Selladurai S (2014) Shape controlled synthesis of CeO2 nanostructures for high performance supercapacitor electrodes. RSC Adv 4:6527–6534.
Maheswari N, Muralidharan G (2015) Supercapacitor behavior of cerium oxide nanoparticles in neutral aqueous electrolytes. Energy Fuels 29:8246–8253.
Alex J, Rajkumar S, PrincyMerlin J, Aravind A, Sajan D, Praveen CS (2021) Single step auto-igniting combustion technique grown CeO2 and Ni-doped CeO2 nanostructures for multifunctional applications. J Alloy Compd 882:160409.
Prasanna K, Santhoshkumar P, Jo YN, Sivagami IN, Kang SH, Joe YC, Lee CW (2018) Highly porous CeO2 nanostructures prepared via combustion synthesis for supercapacitor applications. Appl Surf Sci 449:454–460.
Si L, Wang J, Lu Z, Chen Z, Zhang L, Liu H (2022) In situ construction of CeO2-incorporated hybrid covalent organic frameworks for highly efficient lithium–sulfur batteries. ACS Appl Energy Mater 7:8554–8562.
Chameh B, Khosroshahi N, Bakhtian M, Moradi M, Safarifard V (2022) MOF derived CeO2/CoFe2O4 wrapped by pure and oxidized g-C3N4 sheet as efficient supercapacitor electrode and oxygen reduction reaction electrocatalyst materials. Ceram Int 48:22295–22306.
Talluri B, Yoo K, Kim J (2022) Novel rhombus-shaped cerium oxide sheets as a highly durable methanol oxidation electrocatalyst and high-performance supercapacitor electrode material. Ceram Int 48:164–172.
Omura Y, Yoko A, Seong G, Nishibori M, Ninomiya K, Tomai T, Adschiri T (2022) Uniform organically modified CeO2 nanoparticles synthesized from a carboxylate complex under supercritical hydrothermal conditions: impact of Ce valence. J Phys Chem C 126:6008–6015.
Nazar N, Manzoor S, ur Rehman Y, Bibi I, Tyagi D, Chughtai AH, Gohar RS, Najam-Ul-Haq M, Imran M, Ashiq MN (2022) Metal-organic framework derived CeO2/C nanorod arrays directly grown on nickel foam as a highly efficient electrocatalyst for OER. Fuel 307:121823.
Swathi S, Yuvakkumar R, Senthil Kumar P, Ravi G, Thambidurai M, Dang C, Velauthapillai D (2022) Gadolinium doped CeO2 for efficient oxygen and hydrogen evolution reaction. Fuel 310:122319.
Abdel-Salam AI, Attia SY, El-Hosiny FI, Sadek MA, Mohamed SG, Rashad MM (2022) Facile one-step hydrothermal method for NiCo2S4/rGO nanocomposite synthesis for efficient hybrid supercapacitor electrodes. Mater Chem Phys 277:125554.
Gajraj V, Devi P, Kumar R, Sundriyal N, Mariappan CR (2022) Fabrication of nanocluster-aggregated dense Ce2(MoO4)3 microspherical architectures for high-voltage energy storage and high catalytic energy conversion applications. Energy Fuels 36:7841–7853.
Sahoo S, Kumar R, Joanni E, Singh RK, Shim JJ (2022) Advances in pseudocapacitive and battery-like electrode materials for high performance supercapacitors. J Mater Chem A 10:13190–13240.
Nisa MU, Abid AG, Gouadria S, Munawar T, Alrowaili ZA, Abdullah M, Al-Buriahi MS, Iqbal F, Ehsan MF, Ashiq MN (2022) Boosted electron-transfer/separation of SnO2/CdSe/Bi2S3 heterostructure for excellent photocatalytic degradation of organic dye pollutants under visible light. Surf Interfaces 31:102012.
Fu X, Chen A, Yu Y, Hou S, Liu L (2019) Carbon nanotube@N-doped mesoporous carbon composite material for supercapacitor electrodes. Chem – Asian J 14:634–639.
Wang JG, Zhang Z, Liu X, Wei B (2017) Facile synthesis of cobalt hexacyanoferrate/graphene nanocomposites for high-performance supercapacitor. Electrochim Acta 235:114–121.
Zhang N, Yan X, Li J, Ma J, Ng DHL (2017) Biosorption-directed integration of hierarchical CoO/C composite with nickel foam for high-performance supercapacitor. Electrochim Acta 226:132–139.
Dhelipan M, Arunchander A, Sahu AK, Kalpana D (2017) Activated carbon from orange peels as supercapacitor electrode and catalyst support for oxygen reduction reaction in proton exchange membrane fuel cell. J Saudi Chem Soc 21:487–494.
Liu Y, Liu G, Nie X, Pan A, Liang S, Zhu T (2019) In situ formation of Ni3S2–Cu1.8S nanosheets to promote hybrid supercapacitor performance. J Mater Chem A 7:11044–11052.
Li H, Yang C, Liu F (2009) Novel method for determining stacking disorder degree in hexagonal graphite by X-ray diffraction. Sci China Ser B Chem 52:174–180.
Pop E, Dutton RW, Goodson KE (2004) Analytic band Monte Carlo model for electron transport in Si including acoustic and optical phonon dispersion. J Appl Phys 96:4998.
Wan K, Young JF (1990) Interaction of longitudinal-optic phonons with free holes as evidenced in Raman spectra from Be-doped p-type GaAs. Phys Rev B 41:10772.
Dai F, Zai J, Yi R, Gordin ML, Sohn H, Chen S, Wang D (2014) Bottom-up synthesis of high surface area mesoporous crystalline silicon and evaluation of its hydrogen evolution performance. Nat Commun 5:1–11.
Liu AM, Hidajat K, Kawi S, Zhao DY (2000) A new class of hybrid mesoporous materials with functionalized organic monolayers for selective adsorption of heavy metal ions. Chem Commun 11:1145–1146.
Vinu A, Srinivasu P, Sawant DP, Mori T, Ariga K, Chang JS, Jhung SH, Balasubramanian VV, Hwang YK (2007) Three-dimensional cage type mesoporous CN-based hybrid material with very high surface area and pore volume. Chem Mater 19:4367–4372.
Banda H, Dou JH, Chen T, Libretto NJ, Chaudhary M, Bernard GM, Miller JT, Michaelis VK, Dincǎ M (2021) High-capacitance pseudocapacitors from li+ion intercalation in nonporous, electrically conductive 2D coordination polymers. J Am Chem Soc 143:2285–2292.
Misnon II, Aziz RA, Zain NKM, Vidhyadharan B, Krishnan SG, Jose R (2014) High performance MnO2 nanoflower electrode and the relationship between solvated ion size and specific capacitance in highly conductive electrolytes. Mater Res Bull 57:221–230.
El-Deen AG, Choi JH, Kim CS, Khalil KA, Almajid AA, Barakat NAM (2015) TiO2 nanorod-intercalated reduced graphene oxide as high performance electrode material for membrane capacitive deionization. Desalination 361:53–64.
Chen LF, Huang ZH, Liang HW, Guan QF, Yu SH (2013) Bacterial-cellulose-derived carbon nanofiber@MnO2 and nitrogen-doped carbon nanofiber electrode materials: an asymmetric supercapacitor with high energy and power density. Adv Mater 25:4746–4752.
Li J, Wang N, Tian J, Qian W, Chu W (2018) Cross-coupled macro-mesoporous carbon network toward record high energy-power density supercapacitor at 4 V. Adv Funct Mater 28:1806153.
Fan Z, Yan J, Wei T, Zhi L, Ning G, Li T, Wei F (2011) Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Adv Funct Mater 21:2366–2375.
Li Q, Guo J, Xu D, Guo J, Ou X, Hu Y, Qi H, Yan F (2018) Electrospun N-doped porous carbon nanofibers incorporated with NiO nanoparticles as free-standing film electrodes for high-performance supercapacitors and CO2 capture. Small 14:1704203.
Bibi N, Xia Y, Ahmed S, Zhu Y, Zhang S, Iqbal A (2018) Highly stable mesoporous CeO2/CeS2 nanocomposite as electrode material with improved supercapacitor electrochemical performance. Ceram Int 44:22262–22270.
Ponnaiah SK, Prakash P (2021) A new high-performance supercapacitor electrode of strategically integrated cerium vanadium oxide and polypyrrole nanocomposite. Int J Hydrog Energy 46:19323–19337.
G.S. Kumar, S.A. Reddy, H. Maseed, N.R. Reddy (2020) Facile hydrothermal synthesis of ternary CeO2–SnO2/rGO nanocomposite for supercapacitor application. https://doi.org/10.1142/S1793604720510054.
Nabi G, Ali W, Majid A, Alharbi T, Saeed S, Albedah MA (2022) Controlled growth of Bi-Functional La doped CeO2 nanorods for photocatalytic H2 production and supercapacitor applications. Int J Hydrog Energy 47:15480–15490.
Ghosh S, Anbalagan K, Kumar UN, Thomas T, Rao GR (2020) Ceria for supercapacitors: dopant prediction, and validation in a device. Appl Mater Today 21:100872.
Zhang H, Gu J, Tong J, Hu Y, Guan B, Hu B, Zhao J, Wang C (2016) Hierarchical porous MnO2/CeO2 with high performance for supercapacitor electrodes. Chem Eng J 286:139–149.
Raza W, Nabi G, Shahzad A, Malik N, Raza N (2021) Electrochemical performance of lanthanum cerium ferrite nanoparticles for supercapacitor applications. J Mater Sci Mater Electron 32:7443–7454.
Zhang X, He M, He P, Liu H, Bai H, Chen J, He S, Zhang X, Dong F, Chen Y (2017) Hierarchical structured Sm2O3 modified CuO nanoflowers as electrode materials for high performance supercapacitors. Appl Surf Sci 426:933–943.
Acknowledgements
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R184), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
All authors have contributed equally.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Yes, this article complies with the ethical standards of the journal.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ansari, M.N., Gouadria, S., Khosa, R.Y. et al. Nanospheres type Morphology for regulating the electrochemical property of CeO2 nanostructures for energy storage system. J Sol-Gel Sci Technol 106, 121–130 (2023). https://doi.org/10.1007/s10971-023-06035-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06035-8