Abstract
Integration of corrosion inhibitor-loaded nanocontainers into sol–gel-derived anticorrosion coatings presents a promising strategy to endow them with self-healing ability and improve their long-term corrosion protection properties. In the present work, we demonstrate the preparation of sol–gel anticorrosion coating incorporating benzotriazole-loaded SiO2 mesoporous nanocontainers (BTA@SiO2) synthesized via a modified one-pot method. The nanocontainers exhibited suitable mesoporous structures and pH-dependent inhibitor release properties. Salt spray tests and EIS analysis on the nanocontainer-containing sol–gel coating indicated enhanced corrosion protection properties owing to the high compatibility of the silica skeleton of the nanocontainers with the sol–gel coating matrix, as well as the formation of inhibitive layer on the metal surface.

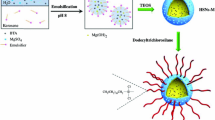
Graphical abstract
Highlights
-
Corrosion inhibitor-loaded silica nanocontainers were prepared via an one-pot approach.
-
The nanocontainers showed satisfactory mesoporous structures and pH-dependent release behavior.
-
The nanocontainer-doped sol–gel coating exhibited enhanced corrosion protection properties.
-
The silica nanocontainer shell provides better compatibility with coating matrix, thus creating less defects.
-
The inhibitive layer formed by the nanocontainers suppressed the corrosion process at coating defects.







Similar content being viewed by others
References
Li X, Zhang D, Liu Z, Li Z, Du C, Dong C (2015) Materials science: share corrosion data. Nature 527(7579):441–442. https://doi.org/10.1038/527441a
Hou B, Li X, Ma X, Du C, Zhang D, Zheng M et al. (2017) The cost of corrosion in China. npj Mater Degrad 1(1):4. https://doi.org/10.1038/s41529-017-0005-2
Hench LL, West JK (1990) The sol-gel process. Chem Rev 90(1):33–72. https://doi.org/10.1021/cr00099a003
Zheludkevich ML, Salvado IM, Ferreira MGS (2005) Sol–gel coatings for corrosion protection of metals. J Mater Chem 15(48):5099–5111. https://doi.org/10.1039/B419153F
Li Y, Wu C, Xue M, Cai J, Huang Y, Yang H (2019) Preparation of sol–gel derived anticorrosive coating on Q235 carbon steel substrate with long-term corrosion prevention durability. Materials 12(12):1960
Guo X, Zhang Q, Ding X, Shen Q, Wu C, Zhang L et al. (2016) Synthesis and application of several sol–gel-derived materials via sol–gel process combining with other technologies: a review. J Sol-Gel Sci Technol 79(2):328–358. https://doi.org/10.1007/s10971-015-3935-6
Chou TP, Chandrasekaran C, Limmer SJ, Seraji S, Wu Y, Forbess MJ et al. (2001) Organic–inorganic hybrid coatings for corrosion protection. J Non-Cryst Solids 290(2):153–162. https://doi.org/10.1016/S0022-3093(01)00818-3
Figueira RB, Silva CJR, Pereira EV (2015) Organic–inorganic hybrid sol–gel coatings for metal corrosion protection: a review of recent progress. J Coat Technol Res 12(1):1–35. https://doi.org/10.1007/s11998-014-9595-6
Yang H, Zhu M, Li Y(2022) Sol–gel research in China: a brief history and recent research trends in synthesis of sol–gel derived materials and their applications. J Sol–Gel Sci Technol https://doi.org/10.1007/s10971-022-05750-y
Guglielmi M (1997) Sol–gel coatings on metals. J Sol-Gel Sci Technol 8(1):443–449. https://doi.org/10.1007/bf02436880
Grigoriev D, Shchukina E, Shchukin DG (2017) Nanocontainers for self-healing coatings. Adv Mater Interfaces 4(1):1600318. https://doi.org/10.1002/admi.201600318
Shchukin D, Möhwald H (2013) A coat of many functions. Science 341(6153):1458–1459. https://doi.org/10.1126/science.1242895
Shchukin DG, Zheludkevich M, Yasakau K, Lamaka S, Ferreira MGS, Möhwald H (2006) Layer-by-Layer assembled nanocontainers for self-healing corrosion protection. Adv Mater 18(13):1672–1678. https://doi.org/10.1002/adma.200502053
Cho SH, White SR, Braun PV (2009) Self-Healing polymer coatings. Adv Mater 21(6):645–649. https://doi.org/10.1002/adma.200802008
Lamaka SV, Vaghefinazari B, Mei D, Petrauskas RP, Höche D, Zheludkevich ML (2017) Comprehensive screening of Mg corrosion inhibitors. Corros Sci 128:224–240. https://doi.org/10.1016/j.corsci.2017.07.011
Finšgar M, Jackson J (2014) Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: a review. Corros Sci 86:17–41. https://doi.org/10.1016/j.corsci.2014.04.044
Tiringer U, Durán A, Castro Y, Milošev I (2018) Self-healing effect of hybrid Sol–Gel coatings based on GPTMS, TEOS, SiO2 nanoparticles and Ce(NO3)3 applied on aluminum alloy 7075-T6. J Electrochem Soc 165(5):C213–C225. https://doi.org/10.1149/2.0211805jes
Zhang F, Ju P, Pan M, Zhang D, Huang Y, Li G et al. (2018) Self-healing mechanisms in smart protective coatings: a review. Corros Sci 144:74–88. https://doi.org/10.1016/j.corsci.2018.08.005
Shchukin DG, Zheludkevich M, Mohwald H (2006) Feedback active coatings based on incorporated nanocontainers. J Mater Chem 16(47):4561–4566. https://doi.org/10.1039/B612547F
Zheludkevich ML, Shchukin DG, Yasakau KA, Möhwald H, Ferreira MGS (2007) Anticorrosion coatings with self-healing effect based on nanocontainers impregnated with corrosion inhibitor. Chem Mater 19(3):402–411. https://doi.org/10.1021/cm062066k
Cai J, Li Y, Wu C, Ding X, Yang H (2020) Preparation and properties of corrosion inhibitor-loaded hollow silica microspheres SiO2. Kuei Suan Jen Hsueh Pao/J Chin Ceram Soc 48(4):584–591. https://doi.org/10.14062/j.issn.0454-5648.20190488
White SR, Sottos NR, Geubelle PH, Moore JS, Kessler MR, Sriram SR et al. (2001) Autonomic healing of polymer composites. Nature 409(6822):794–797. https://doi.org/10.1038/35057232
Pirhady Tavandashti N, Ghorbani M, Shojaei A, Mol JMC, Terryn H, Baert K et al. (2016) Inhibitor-loaded conducting polymer capsules for active corrosion protection of coating defects. Corros Sci 112:138–149. https://doi.org/10.1016/j.corsci.2016.07.003
Haase MF, Grigoriev DO, Möhwald H, Shchukin DG (2012) Development of nanoparticle stabilized polymer nanocontainers with high content of the encapsulated active agent and their application in water-borne anticorrosive coatings. Adv Mater 24(18):2429–2435. https://doi.org/10.1002/adma.201104687
Maia F, Yasakau KA, Carneiro J, Kallip S, Tedim J, Henriques T et al. (2016) Corrosion protection of AA2024 by sol–gel coatings modified with MBT-loaded polyurea microcapsules. Chem Eng J 283:1108–1117. https://doi.org/10.1016/j.cej.2015.07.087
Wei H, Wang Y, Guo J, Shen NZ, Jiang D, Zhang X et al. (2015) Advanced micro/nanocapsules for self-healing smart anticorrosion coatings. J Mater Chem A 3(2):469–480. https://doi.org/10.1039/C4TA04791E
Zheludkevich ML, Tedim J, Ferreira MGS (2012) “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim Acta 82:314–323. https://doi.org/10.1016/j.electacta.2012.04.095
Skorb EV, Fix D, Andreeva DV, Möhwald H, Shchukin DG (2009) Surface-modified mesoporous SiO2 containers for corrosion protection. Adv Funct Mater 19(15):2373–2379. https://doi.org/10.1002/adfm.200801804
Shchukin DG, Möhwald H (2007) Surface-engineered nanocontainers for entrapment of corrosion inhibitors. Adv Funct Mater 17(9):1451–1458. https://doi.org/10.1002/adfm.200601226
Tedim J, Zheludkevich ML, Bastos AC, Salak AN, Lisenkov AD, Ferreira MGS (2014) Influence of preparation conditions of layered double hydroxide conversion films on corrosion protection. Electrochim Acta 117:164–171. https://doi.org/10.1016/j.electacta.2013.11.111
Montemor MF, Snihirova DV, Taryba MG, Lamaka SV, Kartsonakis IA, Balaskas AC et al. (2012) Evaluation of self-healing ability in protective coatings modified with combinations of layered double hydroxides and cerium molibdate nanocontainers filled with corrosion inhibitors. Electrochim Acta 60:31–40. https://doi.org/10.1016/j.electacta.2011.10.078
Zheludkevich ML, Poznyak SK, Rodrigues LM, Raps D, Hack T, Dick LF et al. (2010) Active protection coatings with layered double hydroxide nanocontainers of corrosion inhibitor. Corros Sci 52(2):602–611. https://doi.org/10.1016/j.corsci.2009.10.020
Lvov YM, Shchukin DG, Möhwald H, Price RR (2008) Halloysite clay nanotubes for controlled release of protective agents. ACS Nano 2(5):814–820. https://doi.org/10.1021/nn800259q
Zahidah KA, Kakooei S, Ismail MC, Raja PB (2017) Halloysite nanotubes as nanocontainer for smart coating application: a review. Prog Org Coat 111:175–185. https://doi.org/10.1016/j.porgcoat.2017.05.018
Lvov Y, Wang W, Zhang L, Fakhrullin R (2016) Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv Mater 28(6):1227–1250. https://doi.org/10.1002/adma.201502341
Xu JB, Cao YQ, Fang L, Hu JM (2018) A one-step preparation of inhibitor-loaded silica nanocontainers for self-healing coatings. Corros Sci 140:349–362. https://doi.org/10.1016/j.corsci.2018.05.030
Borisova D, Möhwald H, Shchukin DG (2013) Influence of embedded nanocontainers on the efficiency of active anticorrosive coatings for aluminum alloys part II: influence of nanocontainer position. ACS Appl Mater Interfaces 5(1):80–87. https://doi.org/10.1021/am302141y
Borisova D, Möhwald H, Shchukin DG (2012) Influence of embedded nanocontainers on the efficiency of active anticorrosive coatings for aluminum alloys part I: influence of nanocontainer concentration. ACS Appl Mater Interfaces 4(6):2931–2939. https://doi.org/10.1021/am300266t
Chen T, Chen RP, Jin Z, Liu J (2015) Engineering hollow mesoporous silica nanocontainers with molecular switches for continuous self-healing anticorrosion coating. J Mater Chem A 3(18):9510–9516. https://doi.org/10.1039/c5ta01188d
Shchukina E, Shchukin D, Grigoriev D (2017) Effect of inhibitor-loaded halloysites and mesoporous silica nanocontainers on corrosion protection of powder coatings. Prog Org Coat 102:60–65. https://doi.org/10.1016/j.porgcoat.2016.04.031
Borisova D, Möhwald H, Shchukin DG (2011) Mesoporous silica nanoparticles for active corrosion protection. ACS Nano 5(3):1939–1946. https://doi.org/10.1021/nn102871v
Hollamby MJ, Fix D, Dönch I, Borisova D, Möhwald H, Shchukin D (2011) Hybrid polyester coating incorporating functionalized mesoporous carriers for the holistic protection of steel surfaces. Adv Mater 23(11):1361–1365. https://doi.org/10.1002/adma.201003035
Fickert J, Rupper P, Graf R, Landfester K, Crespy D (2012) Design and characterization of functionalized silica nanocontainers for self-healing materials. J Mater Chem 22(5):2286–2291. https://doi.org/10.1039/C2JM15151K
Liang Y, Wang M, Wang C, Feng J, Li J, Wang L et al. (2016) Facile synthesis of smart nanocontainers as key components for construction of self-healing coating with Superhydrophobic surfaces. Nanoscale Res Lett 11(1):231. https://doi.org/10.1186/s11671-016-1444-3
Snihirova D, Lamaka SV, Cardoso MM, Condeço JAD, Ferreira HECS, de Fatima Montemor M (2014) pH-sensitive polymeric particles with increased inhibitor-loading capacity as smart additives for corrosion protective coatings for AA2024. Electrochim Acta 145:123–131. https://doi.org/10.1016/j.electacta.2014.09.009
Son Y-H, park M, Choy YB, Choi HR, Kim DS, Park KC et al. (2007) One-pot synthetic route to polymer–silica assembled capsule encased with nonionic drug molecule. Chem Commun 27:2799–2801. https://doi.org/10.1039/B702288C
Ramezanzadeh B, Ghasemi E, Askari F, Mahdavian M (2015) Synthesis and characterization of a new generation of inhibitive pigment based on zinc acetate/benzotriazole: solution phase and coating phase studies. Dyes Pigments 122:331–345. https://doi.org/10.1016/j.dyepig.2015.07.013
Viitala R, Jokinen M, Rosenholm JB (2007) Mechanistic studies on release of large and small molecules from biodegradable SiO2. Int J Pharmaceutics 336(2):382–390. https://doi.org/10.1016/j.ijpharm.2006.12.008
Ritger PL, Peppas NA (1987) A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Controlled Release 5(1):37–42. https://doi.org/10.1016/0168-3659(87)90035-6
Peppas NA (1985) Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv 60(4):110–111
Bonora PL, Deflorian F, Fedrizzi L (1996) Electrochemical impedance spectroscopy as a tool for investigating underpaint corrosion. Electrochim Acta 41(7):1073–1082. https://doi.org/10.1016/0013-4686(95)00440-8
Mrad M, Dhouibi L, Montemor MF (2018) Elaboration of γ-glycidoxypropyltrimethoxysilane coating on AA2024-T3 aluminum alloy: influence of synthesis route on physicochemical and anticorrosion properties. Prog Org Coat 121:1–12. https://doi.org/10.1016/j.porgcoat.2018.04.005
Pehkonen SO, Yuan S (2018) Chapter 4 - General background of sol–gel coatings for corrosion mitigation. In: Pehkonen SO, Yuan S (eds). Interface science and technology, Elsevier, p 63–113
Acknowledgements
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ20E020008), by the Open Project of State Key Lab of Silicon Materials, Zhejiang University (SKL2020-11), by the Fundamental Research Funds for the Central Universities (K20220175), and by the Guangxi Department of Science and Technology-Zhejiang University Science, Technology and Innovation Cooperation Project (No. ZD20302002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Y., Zhao, C., Li, Y. et al. Enhanced corrosion resistance and self-healing effect of sol–gel coating incorporating one-pot-synthesized corrosion inhibitor-encapsulated silica nanocontainers. J Sol-Gel Sci Technol 104, 78–90 (2022). https://doi.org/10.1007/s10971-022-05929-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05929-3