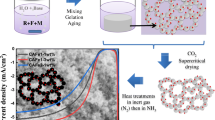
Abstract
Halloysite Nanotubes (HNTs) with large surface/volume ratio and rich reactive groups are incorporated into Fe-based MOF aerogel to develop MOF(Fe)/HNTs composite aerogels for efficient methylene blue (MB) adsorption. A sol–gel method along with supercritical drying technology is applied for the preparation of the MOF(Fe)/HNTs composite aerogels. Micro-morphology and pore structure of the aerogels are properly regulated by controlling the addition amount of HNTs. The obtained samples show three-dimensional mesoporous network structures. The addition of HNTs can slightly increase the specific surface area of the aerogel and then decrease gradually with the increase of HNTs content. The MOF(Fe)/HNTs composite aerogels show favorable adsorption property towards MB and the maximum adsorption capacity can be 384 mg/g with the adsorption efficiency of 96.1%. Besides, the adsorption kinetic analysis reveals that the adsorption process of the MOF(Fe)/HNTs composite aerogel follows the pseudo-second-order model, indicating that chemical sorption may be the rate-limiting step.

Highlights
-
HNTs are successfully employed for the structure regulation of MOF(Fe) aerogel.
-
The MOF(Fe)/HNTs composite aerogel shows favorable methylene blue adsorption property.
-
Chemical sorption tends to be the rate-limiting step of the adsorption process.







Similar content being viewed by others
References
Lu Y, Song S, Wang R, Liu Z, Meng J, Sweetman AJ, Jenkins A, Ferrier RC, Li H, Luo W, Wang T (2015) Environ Int 77:5–15
Mantasha I, Saleh HAM, Qasem KMA, Shahid M, Mehtab M, Ahmad M (2020) Inorg Chim Acta 511:11
Robinson T, McMullan G, Marchant R, Nigam P (2001) Bioresour Technol 77:247–255
Sedighi F, Esmaeili-Zare M, Sobhani-Nasab A, Behpour M (2018) J Mater Sci-Mater El 29:13737–13745
Lim S-H, Rhee S-W (2011) RSC Adv 1.
Pandit P, Basu S (2004) Ind Eng Chem Res 43:7861–7864
Seth S, Savitha G, Moorthy JN (2015) J Mater Chem A 3:22915–22922
Sala M, Lopez-Grimau V, Gutierrez-Bouzan C (2016) Materials 9.
Ghaffar A, Zhang L, Zhu X, Chen B (2018) Environ Sci Technol 52:4265–4274
Li J, Gong J-L, Zeng G-M, Zhang P, Song B, Cao W-C, Liu H-Y, Huan S-Y (2018) J Colloid Interface Sci 527:267–279
Pereira MFR, Soares SF, Órfão JJM, Figueiredo JL (2003) Carbon 41:811–821
Tanthapanichakoon W, Ariyadejwanich P, Japthong P, Nakagawa K, Mukai SR, Tamon H (2005) Water Res 39:1347–1353
Kim S-I, Yamamoto T, Endo A, Ohmori T, Nakaiwa M (2006) Micropor Mesopor Mat 96:191–196
Ho KY, McKay G, Yeung KL (2003) Langmuir 19:3019–3024
Yan Z, Tao S, Yin J, Li G (2006) J Mater Chem 16.
Wu F-C, Tseng R-L, Juang R-S (2005) Sep Purif Technol 47:10–19
Maffei AV, Budd PM, McKeown NB (2006) Langmuir 22:4225–4229
Yaghi OM, O’Keeffe M, Ockwig NW, Chae HK, Eddaoudi M, Kim J (2003) Nature 423:705–714
Kitagawa S, Kitaura R, Noro S (2004) Angew Chem Int Ed Engl 43:2334–2375
Khan NA, Hasan Z, Jhung SH (2013) J Hazard Mater 244-245:444–456
Hwang YK, Hong DY, Chang JS, Jhung SH, Seo YK, Kim J, Vimont A, Daturi M, Serre C, Ferey G (2008) Angew Chem Int Ed Engl 47:4144–4148
Nouar F, Eckert J, Eubank JF, Forster P, Eddaoudi M (2009) J Am Chem Soc 131:2864–2870
Yaghi OM (2016) J Am Chem Soc 138:15507–15509
Karmakar S, Dechnik J, Janiak C, De S (2016) J Hazard Mater 303:10–20
Sun YZ, Chen M, Liu H, Zhu Y, Wang DB, Yan M (2020) Appl Surf Sci 525:9
Huang XX, Qiu LG, Zhang W, Yuan YP, Jiang X, Xie AJ, Shen YH, Zhu JF (2012) Crystengcomm 14:1613–1617
Wan Y, Wang J, Huang F, Xue Y, Cai N, Liu J, Chen W, Yu F (2018) RSC Adv 8:34552–34559
Zhu L, Zong L, Wu X, Li M, Wang H, You J, Li C (2018) ACS Nano 12:4462–4468
Li Y, Zou B, Xiao A, Zhang H (2017) Chin J Chem 35:1501–1511
Wen M, Li G, Liu H, Chen J, An T, Yamashita H (2019) Environ Sci-Nano 6:1006–1025
Ai L, Zhang C, Li L, Jiang J (2014) Appl Catal B-Environ 148-149:191–200
Du M, Guo B, Jia D (2010) Polym Int 59:574–582
Kloprogge JT, Frost RL (1999) J Raman Spectrosc, 30:1079–1085
Jeong GY, Kim Y, Chang S, Kim SJ (2003) Neues Jahrb für Mineralogie - Monatshefte 2003:421–432
Anastopoulos I, Mittal A, Usman M, Mittal J, Yu GH, Nunez-Delgado A, Kornaros M (2018) J Mol Liq 269:855–868
Liu RC, Zhang B, Mei DD, Zhang HQ, Liu JD (2011) Desalination 268:111–116
Liu H, Chu P, Li H, Zhang H, Li J (2016) J Sol-Gel Sci Techn 80:651–659
Song G, Wang Z, Wang L, Li G, Huang M, Yin F (2014) Chin J Catal 35:185–195
Zhang T-Z, Lu Y, Li Y-G, Zhang Z, Chen W-L, Fu H, Wang E-B (2012) Inorg Chim Acta 384:219–224
Dhakshinamoorthy A, Alvaro M, Garcia H (2011) ACS Catal 1:836–840
Tu TH, Cam PTN, Huy LVT, Phong MT, Nam HM, Hieu NH (2019) Mater Lett 238:134–137
Sing KSW (1985) Pure Appl Chem 57:603
Lagergren SYD (1989) Handlingar 24:1–39
Ho YS, McKay G (1999) Process Biochem 34:451–465
Gosset T, Trancart J-L, Thévenot DR (1986) Water Res 20:21–26
Blanchard G, Maunaye M, Martin G (1984) Water Res 18:1501–1507
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51772202).
Author information
Authors and Affiliations
Contributions
HLiu and WY conceived and enabled the research. HLiu performed the material synthesis, physical, and adsorption characterization. HLiu and JC contributed to the experimental results analysis and writing the manuscript. WY contributed to revise the manuscript. CJ, HLi, JL, YL, BZ, and ZC assisted in the result discussion.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, H., Chen, J., Yuan, W. et al. Structure engineering of Fe-based MOF aerogel by Halloysite Nanotubes for efficient methylene blue adsorption. J Sol-Gel Sci Technol 99, 55–62 (2021). https://doi.org/10.1007/s10971-021-05540-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-021-05540-y