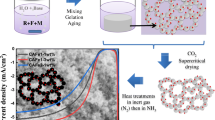
Abstract
The aerogel technique advantageously provides a synthetic route to prepare efficient V–Mg–O catalysts for oxidative dehydrogenation of propane. It was shown that the obtained samples are characterized by a nanocrystal structure with a developed surface area. Such catalysts, comparing with VOx/MgO system prepared by impregnation, demonstrate superior activity in the studied reaction. The main drawback of this system is a shaping difficulty. To get the strong granules, a high molding pressure should be applied that collapses the nanostructure of the aerogels. The addition of alumina powder into the formed sol or before the sol formation stage allowed obtaining the V–Mg–O/γ-Al2O3 catalysts, which can be shaped easily at lower molding pressure. In this case, alumina plays the role of a binder and secondary support. The samples were characterized by low-temperature nitrogen adsorption, scanning and transmission electron microscopies, X-ray diffraction analysis, and X-ray photoelectron spectroscopy. The activity of the prepared samples, in terms of the propylene yield, was compared with the literature data.

Highlights
-
Alumina-supported V–Mg–O catalysts were prepared via aerogel technique.
-
The samples possess nanocrystal structure and developed surface area.
-
Alumina playing the role of a binder facilitates the shaping procedure.
-
The prepared samples exhibit good enhanced activity in oxidative dehydrogenation of propane.









Similar content being viewed by others
References
Park Y-K, Lee CW, Kang NY, Choi WC, Choi S, Oh SH, Park DS (2010) Catalytic cracking of lower-valued hydrocarbons for producing light olefins. Catal Surv Asia 14(2):75–84. https://doi.org/10.1007/s10563-010-9089-1
Bender M (2014) An overview of industrial processes for the production of olefins—C4 hydrocarbons. ChemBioEng Rev 1(4):136–147. https://doi.org/10.1002/cben.201400016
Amghizar I, Vandewalle LA, Van Geem KM, Marin GB (2017) New trends in olefin production. Engineering 3(2):171–178. https://doi.org/10.1016/j.eng.2017.02.006
Sattler JJHB, Ruiz-Martinez J, Santillan-Jimenez E, Weckhuysen BM (2014) Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem Rev 114(20):10613–10653. https://doi.org/10.1021/cr5002436
Wolf M, Raman N, Taccardi N, Haumann M, Wasserscheid P (2020) Coke formation during propane dehydrogenation over Ga−Rh supported catalytically active liquid metal solutions. ChemCatChem 12(4):1085–1094. https://doi.org/10.1002/cctc.201901922
Mamedov EA, Cortés Corberán V (1995) Oxidative dehydrogenation of lower alkanes on vanadium oxide-based catalysts. The present state of the art and outlooks. Appl Catal A-Gen 127(1-2):1–40. https://doi.org/10.1016/0926-860x(95)00056-9
Atanga MA, Rezaei F, Jawad A, Fitch M, Rownaghi AA (2018) Oxidative dehydrogenation of propane to propylene with carbon dioxide. Appl Catal B-Environ 220:429–445. https://doi.org/10.1016/j.apcatb.2017.08.052
Sandupatla AS, Ray K, Thaosen P, Sivananda C, Deo G (2020) Oxidative dehydrogenation of propane over alumina supported vanadia catalyst—effect of carbon dioxide and secondary surface metal oxide additive. Catal Today 354:176–182. https://doi.org/10.1016/j.cattod.2019.06.047
Fan X, Liu D, Zhao Z, Li J, Liu J (2020) Influence of Ni/Mo ratio on the structure-performance of ordered mesoporous Ni-Mo-O catalysts for oxidative dehydrogenation of propane. Catal Today 339:67–78. https://doi.org/10.1016/j.cattod.2019.02.036
Wang L, Ao C, Zhai Y, Feng B, Duan J, Qian S, Zhao W, Zhang L, Liu F (2020) Highly active and stable Co3O4 catalyst for the Low-temperature oxidative dehydrogenation of propane. Inorg Chem Commun 112. https://doi.org/10.1016/j.inoche.2019.107725
Liu J, Hao M, Chen C, Du K, Zhou Q, Zou S, Xiao L, Fan J (2020) Chlorinating CeO2 at surface oxygen vacancies to promote their selectivity in oxidative dehydrogenation of propane to propene. Appl Surf Sci 528. https://doi.org/10.1016/j.apsusc.2020.147025
Soenen V, Herrmann JM, Volta JC (1996) In situ electrical characterization of magnesium vanadate reference phases (meta-MgV2O6, pyro-Mg2V2O7, and ortho-Mg3V2O8) used in oxidative dehydrogenation of propane to propene. J Catal 159(2):410–417. https://doi.org/10.1006/jcat.1996.0104
Balderas-Tapia L, Hernández-Pérez I, Schacht P, Córdova IR, Aguilar-Ríos GG (2005) Influence of reducibility of vanadium–magnesium mixed oxides on the oxidative dehydrogenation of propane. Catal Today 107-108:371–376. https://doi.org/10.1016/j.cattod.2005.07.025
Sugiyama S, Osaka T, Hirata Y, Kondo Y, Nakagawa K, Sotowa K-I (2007) Redox nature of Fe-incorporated magnesium ortho-vanadate as a catalyst for the oxidative dehydrogenation of propane. J Chem Eng Jpn 40(12):1064–1071. https://doi.org/10.1252/jcej.07WE130
Karakoulia SA, Triantafyllidis KS, Lemonidou AA (2008) Preparation and characterization of vanadia catalysts supported on non-porous, microporous and mesoporous silicates for oxidative dehydrogenation of propane (ODP). Micropor Mesopor Mater 110(1):157–166. https://doi.org/10.1016/j.micromeso.2007.10.027
Kootenaei AHS, Towfighi J, Khodadadi A, Mortazavi Y (2014) Stability and catalytic performance of vanadia supported on nanostructured titania catalyst in oxidative dehydrogenation of propane. Appl Surf Sci 298:26–35. https://doi.org/10.1016/j.apsusc.2013.12.172
Chakraborty S, Nayak SC, Deo G (2015) TiO2/SiO2 supported vanadia catalysts for the ODH of propane. Catal Today 254:62–71. https://doi.org/10.1016/j.cattod.2015.01.047
Liu L, Han X, Zhou J, Zhang M, Wu M, Fang K (2017) Oxidative dehydrogenation of propane over three-dimensionally ordered macroporous VMgO catalysts with different vanadium doping. J Porous Mater 25(4):955–963. https://doi.org/10.1007/s10934-017-0507-x
Liu Q, Yang Z, Luo M, Zhao Z, Wang J, Xie Z, Guo L (2019) Vanadium-containing dendritic mesoporous silica nanoparticles: Multifunctional catalysts for the oxidative and non-oxidative dehydrogenation of propane to propylene. Micropor Mesopor Mater 282:133–145. https://doi.org/10.1016/j.micromeso.2019.03.036
Pless J, Bardin BB, Kim H-S, Ko D, Smith MT, Hammond RR, Stair PC, Poeppelmeier KR (2004) Catalytic oxidative dehydrogenation of propane over Mg–V/Mo oxides. J Catal 223(2):419–431. https://doi.org/10.1016/j.jcat.2004.01.023
Klisińska A, Samson K, Gressel I, Grzybowska B (2006) Effect of additives on properties of V2O5/SiO2 and V2O5/MgO catalysts. Appl Catal A-Gen 309(1):10–16. https://doi.org/10.1016/j.apcata.2006.04.028
Lemonidou AA, Machli M (2007) Oxidative dehydrogenation of propane over V2O5-MgO/TiO2 catalystEffect of reactants contact mode. Catal Today 127(1–4):132–138. https://doi.org/10.1016/j.cattod.2007.05.022
Blanco S, Carrazán SRG, Rives V (2008) Oxidative dehydrogenation of propane on Mg-V-Al mixed oxides. Appl Catal A-Gen 342(1-2):93–98. https://doi.org/10.1016/j.apcata.2008.03.002
Kharlamova T, Sushchenko E, Izaak T, Vodyankina O (2016) Phase composition, structural peculiarities and catalytic properties of supported MgO-V2O5/Al2O3 catalysts for oxidative dehydrogenation of propane: Insight into formation of surface Mg-V-O phase. Catal Today 278:174–184. https://doi.org/10.1016/j.cattod.2016.05.006
Zhang S, Liu H (2019) Oxidative dehydrogenation of propane over Mg-V-O oxides supported on MgO-coated silica: Structural evolution and catalytic consequence. Appl Catal A-Gen 573:41–48. https://doi.org/10.1016/j.apcata.2019.01.012
Miranda GP, Ferreira Neto VJM, Young AF, Silveira EB, Pries de Oliveira PG, Mendes FMT (2020) Oxidative dehydrogenation of propane: developing catalysts containing VOX, V-P-O and V-Mg-O species supported on MCM-41 and activated carbon. Catal Today 348:148–156. https://doi.org/10.1016/j.cattod.2019.07.060
Cavani F, Ballarini N, Cericola A (2007) Oxidative dehydrogenation of ethane and propane: how far from commercial implementation? Catal Today 127(1-4):113–131. https://doi.org/10.1016/j.cattod.2007.05.009
Karnaukhov TM, Vedyagin AA, Cherepanova SV, Rogov VA, Stoyanovskii VO, Mishakov IV (2017) Study on reduction behavior of two-component FeMgO oxide system prepared via a sol-gel technique. Int J Hydrog Energ 42(52):30543–30549. https://doi.org/10.1016/j.ijhydene.2017.11.036
Vedyagin AA, Mishakov IV, Karnaukhov TM, Krivoshapkina EF, Ilyina EV, Maksimova TA, Cherepanova SV, Krivoshapkin PV (2017) Sol–gel synthesis and characterization of two-component systems based on MgO. J Sol-Gel Sci Technol 82(2):611–619. https://doi.org/10.1007/s10971-017-4321-3
Karnaukhov TM, Vedyagin AA, Cherepanova SV, Rogov VA, Mishakov IV (2019) Sol–gel synthesis and characterization of the binary Ni–Mg–O oxide system. J Sol-Gel Sci Technol 92(1):208–214. https://doi.org/10.1007/s10971-019-05076-2
Vedyagin AA, Karnaukhov TM, Cherepanova SV, Stoyanovskii VO, Rogov VA, Mishakov IV (2019) Synthesis of binary Co–Mg–O oxide system and study of its behavior in reduction/oxidation cycling. Int J Hydrog Energ 44(37):20690–20699. https://doi.org/10.1016/j.ijhydene.2018.05.044
Ilyina EV, Mishakov IV, Vedyagin AA (2009) Preparation of nanocrystalline VMg(OH)x and VOx·MgO from organometallic precursors. Inorg Mater 45(11):1267–1270. https://doi.org/10.1134/s0020168509110144
Mishakov IV, Ilyina EV, Bedilo AF, Vedyagin AA (2009) Nanocrystalline aerogel VOx/MgO as a catalyst for oxidative dehydrogenation of propane. Reac Kinet Catal Lett 97(2):355–361. https://doi.org/10.1007/s11144-009-0041-1
Mishakov IV, Vedyagin AA, Bedilo AF, Zaikovskii VI, Klabunde KJ (2009) Aerogel VOx/MgO catalysts for oxidative dehydrogenation of propane. Catal Today 144(3-4):278–284. https://doi.org/10.1016/j.cattod.2009.01.018
Ilyina EV, Mishakov IV, Vedyagin AA, Cherepanova SV, Nadeev AN, Bedilo AF, Klabunde KJ (2012) Synthesis and characterization of mesoporous VOx/MgO aerogels with high surface area. Micropor Mesopor Mater 160:32–40. https://doi.org/10.1016/j.micromeso.2012.04.016
Ilyina EV, Mishakov IV, Vedyagin AA, Bedilo AF (2013) Aerogel method for preparation of nanocrystalline CoOx·MgO and VOx·MgO catalysts. J Sol-Gel Sci Technol 68(3):423–428. https://doi.org/10.1007/s10971-013-3012-y
Al-Zahrani SM, Jibril BY, Abasaeed AE (2000) Propane oxidative dehydrogenation over alumina-supported metal oxides. Ind Eng Chem Res 39(11):4070–4074. https://doi.org/10.1021/ie000285o
Choudhary VR, Devadas P, Kinage AK, Guisnet M (1997) Influence of binder on the acidity and performance of H-Gallosilicate (MFI) zeolite in propane aromatization. Appl Catal A-Gen 162(1–2):223–233. https://doi.org/10.1016/s0926-860x(97)00100-2
Lee S-C, Jang J-H, Lee B-Y, Kang M-C, Kang M, Choung S-J (2003) The effect of binders on structure and chemical properties of Fe-K/γ-Al2O3 catalysts for CO2 hydrogenation. Appl Catal A-Gen 253(1):293–304. https://doi.org/10.1016/s0926-860x(03)00540-4
Duan Y, Zhou Y, Sheng X, Zhang Y, Zhou S, Zhang Z (2012) Influence of alumina binder content on catalytic properties of PtSnNa/AlSBA-15 catalysts. Micropor Mesopor Mater 161:33–39. https://doi.org/10.1016/j.micromeso.2012.05.016
Du X, Kong X, Chen L (2014) Influence of binder on catalytic performance of Ni/HZSM-5 for hydrodeoxygenation of cyclohexanone. Catal Commun 45:109–113. https://doi.org/10.1016/j.catcom.2013.10.042
Etim UJ, Bai P, Wang Y, Subhan F, Liu Y, Yan Z (2019) Mechanistic insights into structural and surface variations in Y-type zeolites upon interaction with binders. Appl Catal A-Gen 571:137–149. https://doi.org/10.1016/j.apcata.2018.12.013
Sinha Majumdar S, Celik G, Alexander A-M, Gawade P, Ozkan US (2017) In-situ incorporation of binder during sol-gel preparation of Pd-based sulfated zirconia for reduction of nitrogen oxides under lean-burn conditions: effect on activity and wash-coating characteristics. Appl Catal B-Environ 202:134–146. https://doi.org/10.1016/j.apcatb.2016.08.062
Richards R, Li W, Decker S, Davidson C, Koper O, Zaikovski V, Volodin A, Rieker T, Klabunde KJ (2000) Consolidation of metal oxide nanocrystals. Reactive pellets with controllable pore structure that represent a new family of porous, inorganic materials. J Am Chem Soc 122(20):4921–4925. https://doi.org/10.1021/ja994383g
Fait MJG, Abdallah R, Linke D, Kondratenko EV, Rodemerck U (2009) A novel multi-channel reactor system combined with operando UV/vis diffuse reflectance spectroscopy: proof of principle. Catal Today 142(3-4):196–201. https://doi.org/10.1016/j.cattod.2008.10.023
Rossetti I, Fabbrini L, Ballarini N, Oliva C, Cavani F, Cericola A, Bonelli B, Piumetti M, Garrone E, Dyrbeck H, Blekkan EA, Forni L (2009) V–Al–O catalysts prepared by flame pyrolysis for the oxidative dehydrogenation of propane to propylene. Catal Today 141(3-4):271–281. https://doi.org/10.1016/j.cattod.2008.05.020
Rossetti I, Fabbrini L, Ballarini N, Oliva C, Cavani F, Cericola A, Bonelli B, Piumetti M, Garrone E, Dyrbeck H (2008) V2O5–SiO2 systems prepared by flame pyrolysis as catalysts for the oxidative dehydrogenation of propane. J Catal 256(1):45–61. https://doi.org/10.1016/j.jcat.2008.02.028
Acknowledgements
The authors are grateful to the Russian Ministry of Science and Higher Education (project AAAA-A17-117041710086-6) and the Tomsk Polytechnic University Target Program ‘Science’ (project FSWW-2020-0011). Characterization of the samples was performed using the equipment of the Center of Collective Use “National Center of Catalysts Research.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vedyagin, A.A., Mishakov, I.V. & Ilyina, E.V. A step forward in the preparation of V–Mg–O catalysts for oxidative dehydrogenation of propane. J Sol-Gel Sci Technol 97, 117–125 (2021). https://doi.org/10.1007/s10971-020-05438-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05438-1