Abstract
Niobium pentoxide/silica mesoporous monoliths were prepared by using silicon alkoxide and a bifunctional reactant, ammonium niobate (V) oxalate hydrate (NbOXA), as niobium pentoxide precursor and porogenic agent. The influence of the thermal treatment on the structural, textural characteristics of the samples was evaluated. The thermal stability of the porous network was also evaluated. The introduction of the NbOXA in the silica sol–gel network promoted an increase in pore size diameter and in the total pore volume. The Nb2O5 nanoparticles were well dispersed inside the mesoporous silica matrix and their presence prevented the pore shrinking of the nanocomposite monolith at high temperatures, maintaining the total pore volume of the nanocomposites higher than those of the silica xerogels. To test the availability of the mesoporous nanocomposites as photocatalysts, they were submitted to UV light for methylene blue photobleaching, used as a model waste compound. Only the nanocomposite treated at 900 °C showed morphology and textural proprieties adequate to be photoactive.

Silica/niobium pentoxide composite monoliths with high specific surface area and high pore volume in the range of mesopores, synthesized in one step with high dispersed nanoparticles inside the matrix.
Highlights
-
1.
Ammonium niobium (V) oxalate was used as porogenic agent in the preparation of monoliths of Nb2O5/SiO2 mesoporous nanocomposites.
-
2.
Nb2O5 nanoparticles were well dispersed inside the mesoporous silica matrix.
-
3.
Nb2O5/SiO2 nanocomposites were synthetized with high mesoporosity and good photocatalytic property.
-
4.
The stability of the Nb2O5/SiO2 mesoporous nanocomposites was confirmed by the high specific surface areas obtained at high calcination temperatures.
-
5.
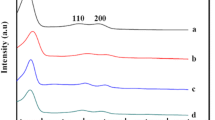
The Nb(V) octahedral coordination of the N2O5 nanoparticles formed inside the nanocomposites was elucidated by EELS.






Similar content being viewed by others
References
Brandão RF, Quirino RL, Mello VM, Tavares AP, Peres AC, Guinhos F et al. (2009) Synthesis, characterization and use of Nb2O5 based caalysts in producing biofuels by transesterification, esterification and pyrolysis. J Braz Chem Soc 20:954–66
Turco R, Aronne A, Carniti P, Gervasini A, Minieri L, Pernice P et al. (2015) Influence of preparation methods and structure of niobium oxide-based catalysts in the epoxidation reaction. Catal Today 254:99–103
Athar T, Hashmi A, Al-Hajry A, Ansari ZA, Ansari SG (2012) One-pot synthesis and characterization of Nb2O5 nanopowder. J Nanosci Nanotechnol 12:7922–6
Jaramillo-Páez C, Sánchez-Fernández FJ, Navío JA, Hidalgo MC (2018) Photo-induced processes on Nb 2 O 5 synthesized by different procedures. J Photochemistry Photobiol A: Chem 359:40–52
Ziolek M, Sobczak I (2017) The role of niobium component in heterogeneous catalysts. Catal Today 285:211–25
Umpierres CS, Prola LD, Adebayo MA, Lima EC, Dos Reis GS, Kunzler DD et al. (2017) Mesoporous Nb2O5/SiO2 material obtained by sol-gel method and applied as adsorbent of crystal violet dye. Environ Technol 38:566–78
Francisco MSP, Gushikem Y (2002) Synthesis and characterization of SiO2–Nb2O5systems prepared by the sol–gel method: structural stability studies. J Mater Chem 12:2552–8
Anilkumar M, Hoelderich WF (2012) Gas phase Beckmann rearrangement of cyclohexanone oxime to ɛ-caprolactam over mesoporous, microporous and amorphous Nb2O5/silica catalysts: a comparative study. Catal Today 198:289–99
Gao X, Wachs IE, Wong MS, Ying JY (2001) Structural and reactivity properties of Nb MCM-41: comparison with that of highly dispersed Nb2O5/SiO2 catalysts. J Catal 203:18–24
Yoshida H, Tanaka T, Yoshida T, Funabiki T, Yoshida S (1996) Control of the structure of niobium oxide species on silica by the equilibrium adsorption method. Catal Today 28:79–89
He J, Li Q-J, Fan Y-N (2013) Dispersion states and acid properties of SiO2-supported Nb2O5. J Solid State Chem 202:121–7
Sachse A, Galarneau A, Coq B, Fajula F (2011) Monolithic flow microreactors improve fine chemicals synthesis. New J Chem 35:259
Su TT, Zhai YC, Jiang H, Gong H (2009) Studies on the thermal decomposition kinetics and mechanism of ammonium niobium oxalate. J Therm Anal Calorim 98:449
Bach D, Schneider R, Gerthsen D, Verbeeck J, Sigle W (2009) EELS of niobium and stoichiometric niobium-oxide phases—Part I: plasmon and near-edges fine structure. Microsc Microanalysis 15:505–23
Bach D, Störmer H, Schneider R, Gerthsen D, Verbeeck J (2006) EELS investigations of different niobium oxide phases. Microsc Microanal 12:416–23
Nico C, Monteiro T, Graça MPF (2016) Niobium oxides and niobates physical properties: review and prospects Prog Mater Sci 80:1–37
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J et al. (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–69
Brinker CJ, Scherer GW (1990) Sol-Gel Science: the physics and chemistry of sol-gel processing. Academic Press, San Diego, CA
de Sousa EM, de Sousa AP, Mohallem ND, Lago RM (2003) Copper-silica sol-gel catalysts: structural changes of Cu species upon thermal treatment. J Sol-Gel Sci Technol 26:873–7
Silva Â, Wilson K, Lee AF, dos Santos VC, Cons Bacilla AC, Mantovani KM et al. (2017) Nb 2 O 5 /SBA-15 catalyzed propanoic acid esterification. Appl Catal B: Environ 205:498–504
Liu B, Zhao X, Terashima C, Fujishima A, Nakata K (2014) Thermodynamic and kinect analysis of heterogeneous photocatalysis for semiconductor systems. Phys Chem Chem Phys 16:8751–60
Acknowledgements
This work was supported by CNPq, CAPES, and FAPEMIG (Brazilian agencies). The authors acknowledge Center of Microscopy at UFMG for the infrastructure.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lima, L.F.d.S., Coelho, C.R., Gomes, G.H.M. et al. Nb2O5/SiO2 mesoporous monoliths synthetized by sol–gel process using ammonium niobate oxalate hydrate as porogenic agent. J Sol-Gel Sci Technol 93, 168–174 (2020). https://doi.org/10.1007/s10971-019-05146-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-05146-5